Examples Research

Our Publications
Since 2014, several publications have emerged from our core research
and from the collaboration with our partners.
Core research
Graphical abstracts on this page are copyright protected.
Perspective
“An oscillating reaction to produce clean fuels”
J.W. Niemantsverdriet, and C.J. Weststrate, Science 382 (2023) 35-36
Related research article
“The oscillating Fischer-Tropsch reaction”; R. Zhang, Y. Wang, P. Gaspard, N. Kruse, Science 382 (2023) 99-103
Editor’s summary
Reaction temperature and product formation oscillate during the hydrogenation of carbon monoxide to form hydrocarbons (Fischer-Tropsch synthesis) over cobalt–ceria catalysts. Zhang et al. observed long-duration oscillations of several degrees Celsius with a period of several minutes for a 1:1 reactant feed over cobalt–cerium oxide powder catalyst operating at 220°C, as well as changes in product formation and reaction rates (see the Perspective by Niemantsverdriet and Westrate). A kinetic model including a carbon monoxide insertion step, thermal activation of carbon–oxygen bonds, and periodic temperature forcing could account for the observations. —Phil Szuromi
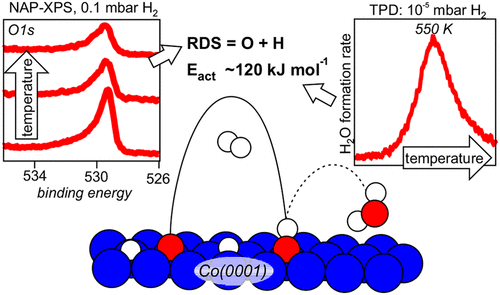
“Water Formation Kinetics on Co(0001) at Low and Near-Ambient Hydrogen Pressures in the Context of Fischer–Tropsch Synthesis”
C.J. Weststrate, D. Sharma, D. García Rodríguez, H.O.A. Fredriksson, and J.W. Niemantsverdriet, J. Phys. Chem. C 127 (2023) 3452–3461
Abstract
Understanding the kinetics of oxygen removal from catalytically active metal surfaces by hydrogen is important for several catalytic reactions such as Fischer–Tropsch synthesis, methanation of CO or CO2, and the reverse-water–gas-shift reaction. Motivated by FTS, a Co(0001) single crystal model catalyst was used to study the kinetics of oxygen removal through reaction with hydrogen. Kinetic studies in the 10–7–10–4 mbar H2 pressure regime show that water formation is first order in the surface hydrogen concentration while the order in oxygen concentration changes from one at low oxygen coverage to zero at high oxygen coverage. In situ XPS shows that the hydrogen surface concentration saturates around 10–1 mbar and on this basis the typical temperature of 450 K needed for water formation in this pressure regime can be considered as typical for high pressures as well. The absence of OH buildup during the reaction points to O + H as the rate-limiting step, with a barrier of ∼120 kJ mol–1. Such a high barrier shows that slow removal of adsorbed oxygen from the surface of reactive metal catalysts such as cobalt may be rate-limiting for the overall reaction.
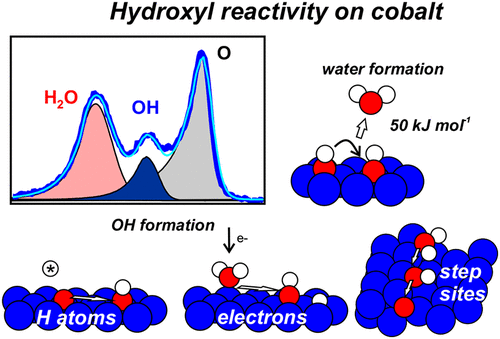
“Water and Hydroxyl Reactivity on Flat and Stepped Cobalt Surfaces”
C.J. Weststrate, D. Sharma, M.A. Gleeson, and J.W. Niemantsverdriet, J. Phys. Chem. C 127 (2023) 2974–2980
Abstract
Hydroxyl adsorbates generally appear as transient species during water formation from adsorbed oxygen and hydrogen atoms on a metal surface, a reaction that is part of the catalytic cycle in various important surface-catalyzed reactions such as Fischer–Tropsch synthesis. In the present work, temperature-programmed desorption and in situ synchrotron XPS were used to study water adsorption and OH reactivity on a flat and a stepped cobalt single crystal surface. Water adsorbs intact on the flat Co(0001) surface and desorbs around 160 K. Electrons induce dissociation of water and produce OH species at low temperature. Hydroxyl species can also be formed by the reaction between Oad and H2O, but only for high initial oxygen coverage while low coverage Oad appears largely unreactive. Reactive hydrogen species (H atoms) produced by a hot tungsten filament hydrogenate adsorbed oxygen atoms at low temperature already and both OHad and H2O are formed. In all cases, hydroxyl adsorbates react around 190 K to form water via 2 OHad → H2O (g) + Oad associated with an activation barrier of 40–50 kJ mol–1. Water readily dissociates on the step sites exposed by vicinal Co(10–19). A part of the OHad species recombine to form water and oxygen between 200 and 300 K, while decomposition of OHad into Oad and Had dominates above 370 K. For catalysis, the high reactivity of step sites for water dissociation and the high stability of OHad at these sites implies that O removal from these sites may be difficult and may limit the overall rate of Fischer–Tropsch synthesis on cobalt catalysts.
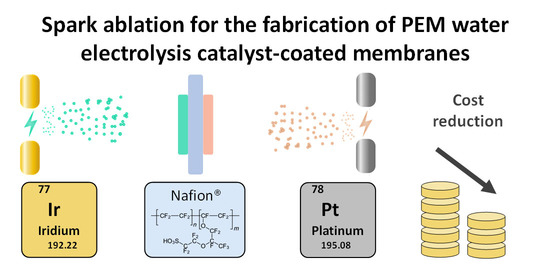
“Spark Ablation for the Fabrication of PEM Water Electrolysis Catalyst-Coated Membranes”
F.M. Sapountzi, M. Lavorenti, W. Vrijburg, S. Dimitriadou, B. Tyburska-Pueschel, P. Thüne, J.W. Niemantsverdriet, T.V. Pfeiffer, M.N. Tsampas, Catalysts 12 (2022) 1343
Abstract
Proton-exchange-membrane (PEM) electrolyzers represent a promising technology for sustainable hydrogen production, owing to their efficiency and load flexibility. However, the acidic nature of PEM demands the use of platinum-group metal-electrocatalysts. Apart from the associated high capital costs, the scarcity of Ir hinders the large-scale implementation of the technology. Since low-cost replacements for Ir are not available at present, there is an urgent need to engineer catalyst-coated membranes (CCMs) with homogeneous catalyst layers at low Ir loadings. Efforts to realize this mainly rely on the development of advanced Ir nanostructures with maximized dispersion via wet chemistry routes. This study demonstrates the potential of an alternative vapor-based process, based on spark ablation and impaction, to fabricate efficient and durable Ir- and Pt-coated membranes. Our results indicate that spark-ablation CCMs can reduce the Ir demand by up to five times compared to commercial CCMs, without a compromise in activity. The durability of spark-ablation CCMs has been investigated by applying constant and dynamic load profiles for 150 h, indicating different degradation mechanisms for each case without major pitfalls. At constant load, an initial degradation in performance was observed during the first 30 h, but a stable degradation rate of 0.05 mV h−1 was sustained during the rest of the test. The present results, together with manufacturing aspects related to simplicity, costs and environmental footprint, suggest the high potential of spark ablation having practical applications in CCM manufacturing.
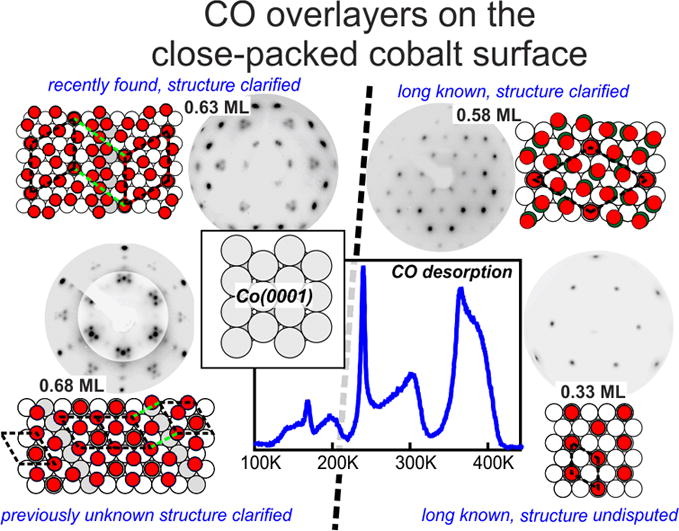
“CO adsorption on Co(0001) revisited: High-coverage CO superstructures on the close-packed surface of cobalt”
C.J. Weststrate, J.W. Niemantsverdriet, J. Catal. 408 (2022) 142-154
Abstract
The adsorbate overlayer structures formed by CO on highly covered Co(0001) were investigated using temperature programmed desorption, low energy electron diffraction, reflection absorption infrared spectroscopy and synchrotron-based x-ray photoemission spectroscopy, with the purpose to clarify some recent confusion on CO adsorption at high coverages. TPD shows that the coverage of the (2√3 × 2√3)R30o structure (abbreviated as 2√3 structure) is 7/12 (0.583) ML. Its unit cell contains one COtop and six CObridge species that form three ‘bridge dicarbonyls’ in which two CObridge adsorbates share the same cobalt surface atom. Between 0.58 and 0.63 ML the 2√3 structure breaks up into small 2D domains separated by antiphase domain boundaries that consist of ‘double bridge dicarbonyls’ in which three CObridge adsorbates share two cobalt surface atoms. A phase transition that occurs around 0.63 ML creates previously unknown c(8 × 2) and c(12 × 2) domain boundary structures which consist of high density strips with a (2 × 2)–3CO structure and top/hollow site occupation which are separated by antiphase domain boundaries with a lower local coverage. Up to 0.63 ML the structures found at low temperature in UHV are very similar to those observed by others using in-situ STM at 300 K under CO pressure. This changes above 0.63 ML, where STM shows that moiré structures form instead of the c(n × 2) domain boundary structures. We propose that the moiré and c(n × 2) structures have a very similar enthalpy of formation so that small variations of temperature and CO chemical potential can lead to different structures for the same CO coverage. We show that these high-density phases that exist above 0.5 ML do not form at the typical pressures and temperatures used in applied FTS, but they do need to be considered when CO adsorption at room temperature is used as a diagnostic tool to characterize the catalyst surface.
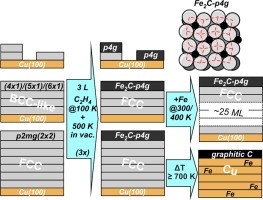
“Iron carbide formation on thin iron films grown on Cu(100): FCC iron stabilized by a stable surface carbide”
D. García Rodríguez, M.A. Gleeson, J.V. Lauritsen, Z. Li, X. Yu, J.W. Niemantsverdriet, C.J. Weststrate, Appl. Surf. Sci. 585 (2022) 152684
Abstract
The adsorbate overlayer structures formed by CO on highly covered Co(0001) were investigated using temperature programmed desorption, low energy electron diffraction, reflection absorption infrared spectroscopy and synchrotron-based x-ray photoemission spectroscopy, with the purpose to clarify some recent confusion on CO adsorption at high coverages. TPD shows that the coverage of the (2√3 × 2√3)R30o structure (abbreviated as 2√3 structure) is 7/12 (0.583) ML. Its unit cell contains one COtop and six CObridge species that form three ‘bridge dicarbonyls’ in which two CObridge adsorbates share the same cobalt surface atom. Between 0.58 and 0.63 ML the 2√3 structure breaks up into small 2D domains separated by antiphase domain boundaries that consist of ‘double bridge dicarbonyls’ in which three CObridge adsorbates share two cobalt surface atoms. A phase transition that occurs around 0.63 ML creates previously unknown c(8 × 2) and c(12 × 2) domain boundary structures which consist of high density strips with a (2 × 2)–3CO structure and top/hollow site occupation which are separated by antiphase domain boundaries with a lower local coverage. Up to 0.63 ML the structures found at low temperature in UHV are very similar to those observed by others using in-situ STM at 300 K under CO pressure. This changes above 0.63 ML, where STM shows that moiré structures form instead of the c(n × 2) domain boundary structures. We propose that the moiré and c(n × 2) structures have a very similar enthalpy of formation so that small variations of temperature and CO chemical potential can lead to different structures for the same CO coverage. We show that these high-density phases that exist above 0.5 ML do not form at the typical pressures and temperatures used in applied FTS, but they do need to be considered when CO adsorption at room temperature is used as a diagnostic tool to characterize the catalyst surface.
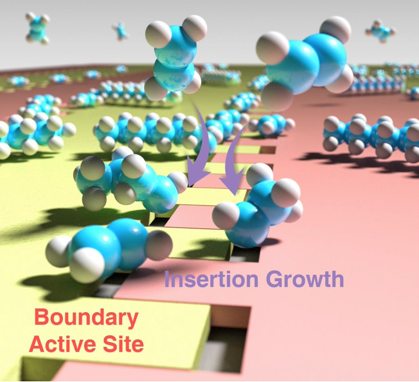
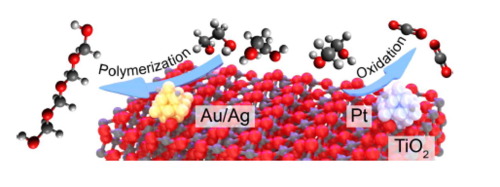
“Visualization of on-surface ethylene polymerization through ethylene insertion”
W. Guo, J. Yin, Z. Xu, W. Li, Z. Peng, C.J. Weststrate, X. Yu, Y. He, Z. Cao, X. Wen, Y. Yang, K. Wu, Y. Li, J.W. Niemantsverdriet, X. Zhou, Science 375 (2022) 1188-1191
Abstract
Polyethylene production through catalytic ethylene polymerization is one of the most common processes in the chemical industry. The popular Cossee-Arlman mechanism hypothesizes that the ethylene be directly inserted into the metal–carbon bond during chain growth, which has been awaiting microscopic and spatiotemporal experimental confirmation. Here, we report an in situ visualization of ethylene polymerization by scanning tunneling microscopy on a carburized iron single-crystal surface. We observed that ethylene polymerization proceeds on a specific triangular iron site at the boundary between two carbide domains. Without an activator, an intermediate, attributed to surface-anchored ethylidene (CHCH3), serves as the chain initiator (self-initiation), which subsequently grows by ethylene insertion. Our finding provides direct experimental evidence of the ethylene polymerization pathway at the molecular level.
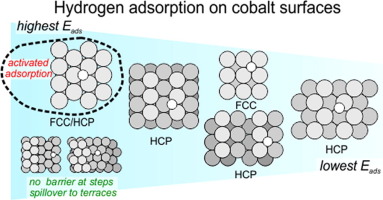
“Structure-dependent adsorption and desorption of hydrogen on FCC and HCP cobalt surfaces”
C.J. Weststrate, D. Garcia Rodriguez, D. Sharma, J.W. Niemantsverdriet, J. Catal. 405 (2022) 303–312
Abstract
The interaction of hydrogen with cobalt surfaces is of fundamental interest for Fischer-Tropsch synthesis. In the present work, the adsorption and desorption of hydrogen was studied on various cobalt single crystal surfaces that together represent the surface structures exposed by FCC and HCP cobalt nanoparticles used in applied catalysis. Dissociative hydrogen adsorption is activated on flat Co(0001), especially for hydrogen coverages beyond 0.5 ML. A tungsten filament creates hydrogen atoms and hot hydrogen molecules that increase the dissociative sticking probability and make it possible to obtain hydrogen coverages above 0.5 ML. Hydrogen in excess of 0.5 ML binds more weakly and desorbs in a separate low temperature desorption peak, in line with theoretical predictions. A third desorption peak appears above 1 ML and is attributed to subsurface hydrogen, the formation of which is attributed to hydrogen atoms produced by the tungsten filament. Adsorbed hydrogen atoms form (islands) of an ordered (2 × 2)-2H honeycomb structure for coverages between 0.3 and 0.8 ML which points to a specific stability of this structure. Step and kink sites on vicinal close-packed surfaces provide a low energy path for both hydrogen adsorption and desorption which results in a much higher dissociative sticking probability and a lower desorption temperature. The hydrogen adsorption strength on various FCC and HCP cobalt surfaces varies between 30 and 45 kJ/mol Had and is strongest on threefold hollow sites on the close-packed terraces while it is significantly lower on fourfold hollow sites on FCC-(100) and on threefold hollow sites on various open HCP surfaces. Under reaction conditions, the structure-dependent adsorption energy translates to a two to three orders of magnitude variation of the equilibrium constant for hydrogen.
“Novel microreactor and generic model catalyst platform for the study of fast temperature pulsed operation – CO oxidation rate enhancement on Pt”
Z. Zhu, M. Weber, M.A, Verheijen, A.A. Bol, V. Spinu, L. Ozkan, A.C.P.M. (Ton) Backx, J.W. (Hans) Niemantsverdriet, H.O.A. Fredriksson, Chemical Engineering Journal 425 (2021) 131559
Abstract
A novel setup to study the effect of fast, high temperature pulses on catalytic reactions has been developed. The system is based on a microreactor and a generic catalyst support, with an integrated heater that allows for temperature changes up to 230 °C within 20–30 µs and temperature measurements with µs time resolution. Standard microfabrication techniques were used to prepare the catalyst support and the heater element, a 100 nm thick Pt film on a SiO2 terminated Si wafer. Atomic layer deposition (ALD) was used to cover the Pt film with a uniform Al2O3 nanolayer, making sure it solely works as a heater. The Al2O3 terminated wafers could thus be used as a generic platform for the study of supported catalysts under conditions where extremely fast temperature changes occur. ALD was then further used to deposit Pt nanoparticles of various sizes on the Al2O3 surface, serving as model catalysts. CO oxidation was chosen as the test reaction to investigate the concept of temperature pulsed operation and the results show that reaction rates can be accurately controlled and increased significantly by the application of fast, well-controlled temperature pulses, while the power input required to drive the reaction is lower than for steady state operation. Simulations of the reaction kinetics show that reactants desorb from the surface during the fast, high temperature pulses and that the reaction rate enhancement takes place mainly during the relatively slow re-population of the catalyst surface after the temperature pulse.
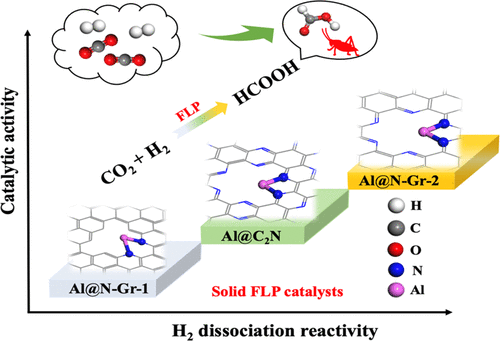
“Silica Nanopowder Supported Frustrated Lewis Pairs for CO2 Capture and Conversion to Formic Acid“
K. Mentoor, L. Twigge, J.W. (Hans) Niemantsverdriet, J.C. Swarts, E.H.G. Erasmus, Inorganic Chemistry 60 (2021) 55-69
Abstract
Developing efficient and inexpensive main group catalysts for CO2 conversion and utilization has attracted increasing attention, as the conversion process would be both economical and environmentally benign. Here, based on the main group element Al, we designed several heterogeneous frustrated Lewis acid/base pair (FLP) catalysts and performed extensive first-principles calculations for the hydrogenation of CO2. These catalysts, including Al@N-Gr-1, Al@N-Gr-2, and Al@C2N, are composed of a single Al atom and two-dimensional (2D) N-doped carbon-based materials to form frustrated Al/C or Al/N Lewis acid/base pairs, which are all predicted to have high reactivity to absorb and activate hydrogen (H2). Compared with Al@N-Gr-1, both Al@N-Gr-2 and Al@C2N, especially Al@N-Gr-2, containing Al/N Lewis pairs exhibit better catalytic activity for CO2 hydrogenation with lower activation energies. CO2 hydrogenation on the three catalysts prefers to go through a three-step mechanism, i.e., the heterolytic dissociation of H2, followed by the transfer of the hydride near Al to CO2, and finally the activation of a second H2 molecule. Other IIIA group element (B and Ga)-embedded N-Gr-2 materials (B@N-Gr-2 and Ga@N-Gr-2) were also explored and compared. Both Al@N-Gr-2 and Ga@N-Gr-2 show higher catalytic activity for CO2 hydrogenation to HCOOH than B@N-Gr-2. However, the CO2 hydrogenation path on Ga@N-Gr-2 tends to follow a two-step mechanism, including H2 dissociation and subsequent hydrogen transfer. The present study provides a potential solution for CO2 hydrogenation by designing novel and effective FLP catalysts based on main group elements.
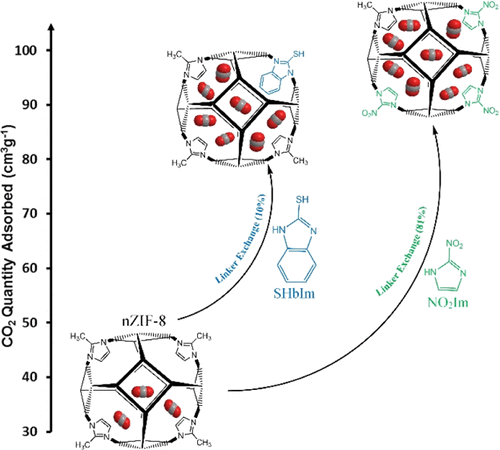
“Optimized CO2 Capture of the Zeolitic Imidazolate Framework ZIF-8 Modified by Solvent-Assisted Ligand Exchange”
Y.W. Abraha, C.-W. Tsai, J.W. (Hans) Niemantsverdriet, E.H.G. Langner, ACS Omega 6 (2021) 21850–21860
Abstract
Zeolitic imidazolate frameworks, like ZIF–8 and related structures, have shown great potential for the capture of carbon dioxide. Modifying their structure by exchanging part of the constituent organic ligands is a proven method for enhancing the capacity to absorb CO2. In this work, we performed solvent–assisted ligand exchange (SALE) on nanosized ZIF–8 (nZIF-8) with a series of functionalized imidazole derivatives (exchange percentages, after 24 h): 2-bromoimidazole (19%), 2-chloroimidazole (29%), 2-trifluoromethylbenzimidazole (4%), 2-mercaptobenzimidazole (4%), and 2-nitroimidazole (54%). The sodalite topology and porosity of nZIF-8 were maintained with all SALE modifications. Low-pressure CO2 adsorption of nZIF-8 (38.5 cm3 g–1) at STP was appreciably enhanced with all mixed-linker SALE products. Using halogenated (−Cl, −Br, and −CF3) imidazole derivatives in a 24 h SALE treatment resulted in increases between 11 and 22% in CO2 adsorption, while the thiol (−SH)- and nitro (−NO2)-functionalized SALE products led to 32 and 100% increases in CO2 uptakes, respectively. These CO2 uptakes were further optimized by varying the SALE treatment time. The SHbIm- and NO2Im-exchanged SALE products of nZIF-8 show 87 and 98 cm3 g–1 of CO2 uptakes after 60 and 120 h of SALE, respectively. These are record high CO2 adsorptions for all reported ZIF derivatives at low-pressure conditions.
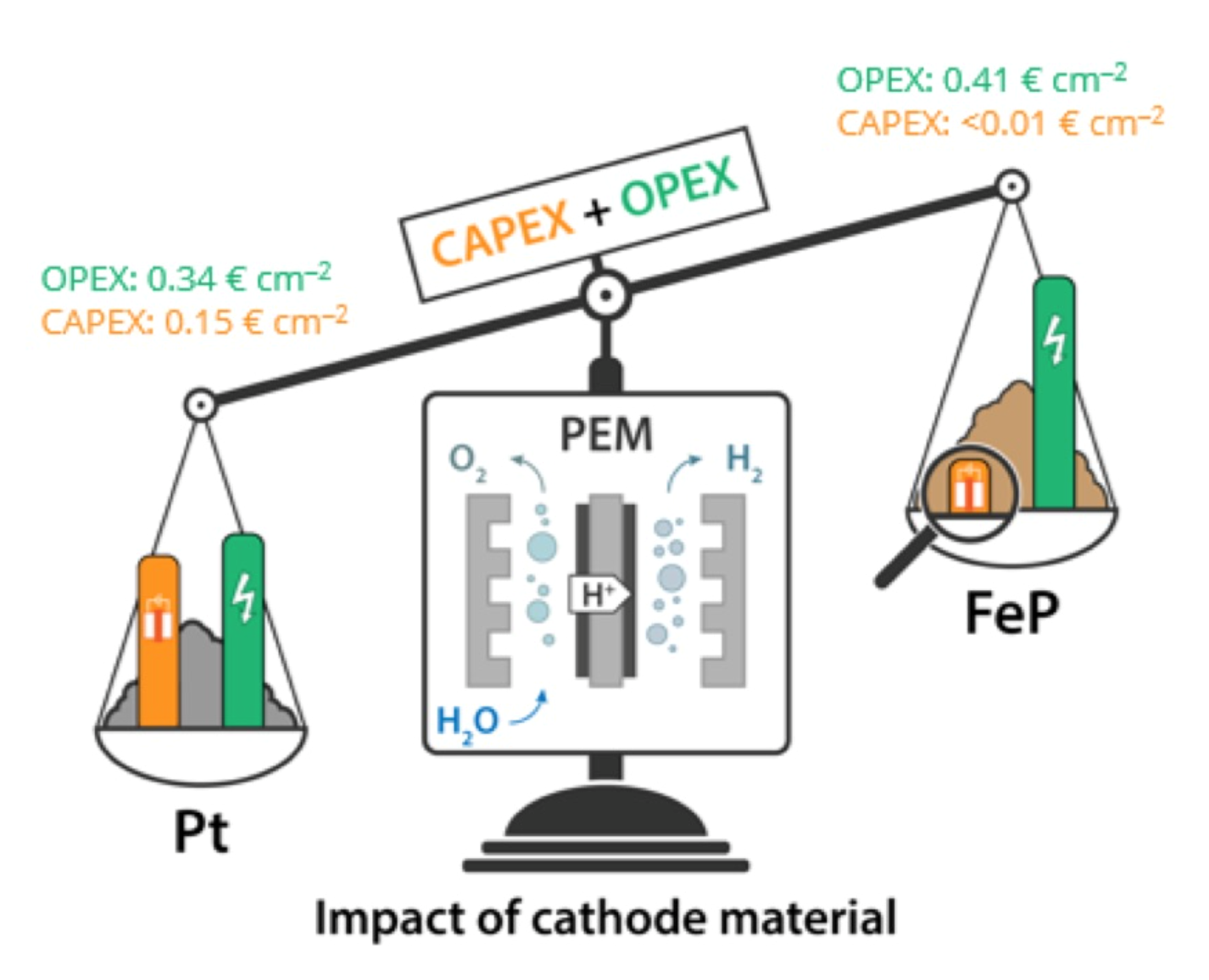
“FeP Nanocatalyst with Preferential [010] Orientation Boosts the Hydrogen Evolution Reaction in Polymer-Electrolyte Membrane Elektrolyzer”
F.M. Sapountzi, E.D. Orlova, J.P.S. Sousa, L.M. Salonen, O.I. Lebedev, G. Zafeiropoulos, M.N. Tsampas, J.W. (Hans) Niemantsverdriet, and Y.V. Kolen’ko, Energy Fuels 34 (2020) 6423–6429
Abstract
The development of nonprecious metal electrocatalysts for polymer-electrolyte membrane (PEM) water electrolysis is a milestone for the technology, which currently relies on rare and expensive platinum-group metals. Half-cell measurements have shown iron phosphide materials to be promising alternative hydrogen evolution electrocatalysts, but their realistic performance in flow-through devices remains unexplored. To fill this gap, we report herein the activity and durability of FeP nanocatalyst under application-relevant conditions. Our facile synthesis route proceeds via impregnation of an iron complex on conductive carbon support followed by phosphorization, giving rise to highly crystalline nanoparticles with predominantly exposed [010] facets, which accounts for the high electrocatalytic activity. The performance of FeP gas diffusion electrodes toward hydrogen evolution was examined under application-relevant conditions in a single cell PEM water electrolysis at 22 °C. The FeP cathode exhibited a current density of 0.2 A cm–2 at 2.06 V, corresponding to a difference of merely 0.07 W cm–2 in power input as compared to state-of-the-art Pt cathode, while outperforming other nonprecious cathodes operated at similar temperature. Quantitative product analysis of our PEM device excluded the presence of side reactions and provided strong experimental evidence that our cell operates with 84–100% Faradaic efficiencies and with 4.1 kWh Nm–3 energy consumption. The FeP cathodes exhibited stable performance of over 100 h at constant operation, while their suitability with the intermittency of renewable sources was demonstrated upon 36 h operation at variable power inputs. Overall, the performance as well as our preliminary cost analysis reveal the high potential of FeP for practical applications.
“Mechanistic insight into carbon-carbon bond formation on cobalt under simulated Fischer-Tropsch synthesis conditions“
C.J. (Kees-Jan) Weststrate, D. Sharma, D. Garcia Rodriguez, M.A. Gleeson, H.O.A. Fredriksson & J.W. (Hans) Niemantsverdriet, Nature Communications 11 (2020) Article number: 750
Abstract
Facile C-C bond formation is essential to the formation of long hydrocarbon chains in Fischer-Tropsch synthesis. Various chain growth mechanisms have been proposed previously, but spectroscopic identification of surface intermediates involved in C-C bond formation is scarce. We here show that the high CO coverage typical of Fischer-Tropsch synthesis affects the reaction pathways of C2Hx adsorbates on a Co(0001) model catalyst and promote C-C bond formation. In-situ high resolution x-ray photoelectron spectroscopy shows that a high CO coverage promotes transformation of C2Hx adsorbates into the ethylidyne form, which subsequently dimerizes to 2-butyne. The observed reaction sequence provides a mechanistic explanation for CO-induced ethylene dimerization on supported cobalt catalysts. For Fischer-Tropsch synthesis we propose that C-C bond formation on the close-packed terraces of a cobalt nanoparticle occurs via methylidyne (CH) insertion into long chain alkylidyne intermediates, the latter being stabilized by the high surface coverage under reaction conditions.
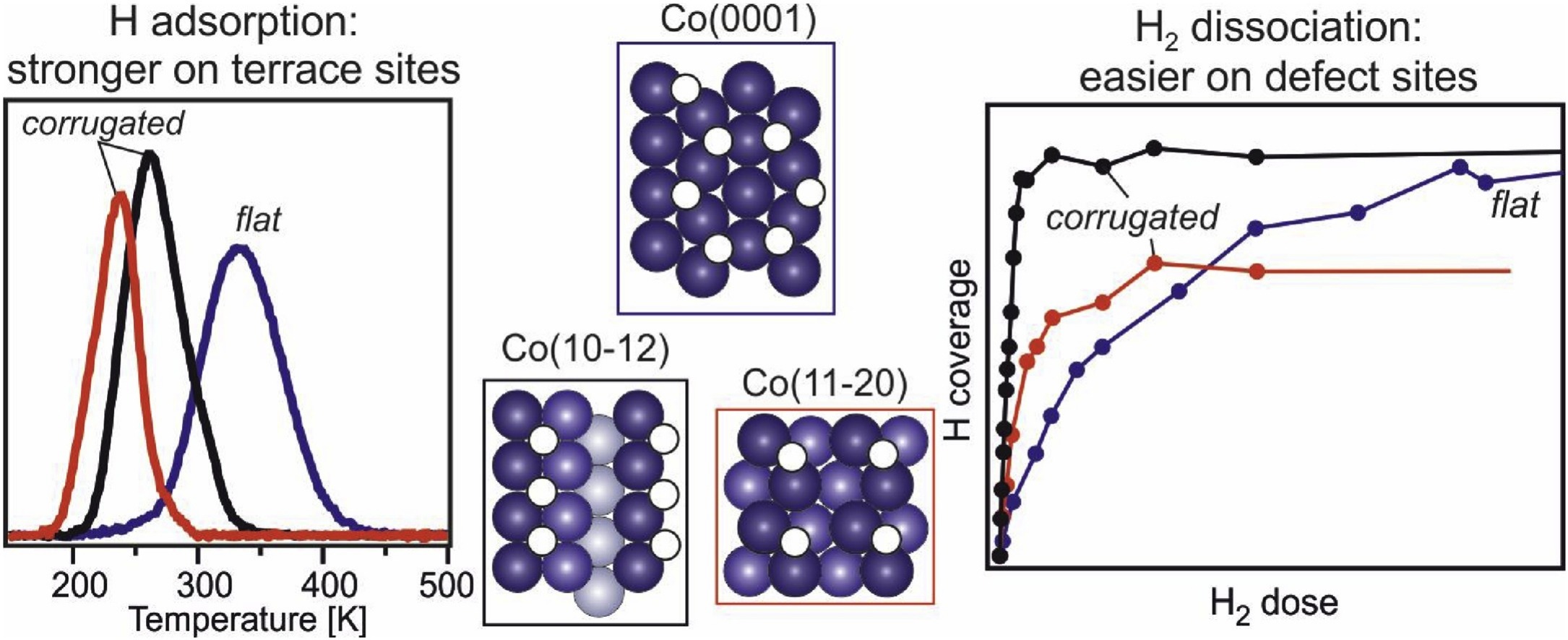
“Interaction of hydrogen with flat (0001) and corrugated (11–20) and (10–12) cobalt surfaces: Insights from experiment and theory”
C.J. (Kees-Jan) Weststrate, M. Mahmoodinia, M.H. Farstad, I.-H. Svenum, M.D. Strømsheim, J.W. (Hans) Niemantsverdriet, H.J. Venvik, Catalysis Today 342 (2020) 124-130
Abstract
Cobalt catalysts are used on a commercial scale to produce synthetic fuels via the Fischer-Tropsch synthesis process. As adsorbed hydrogen atoms are involved in many of the elementary reaction steps that occur on the catalyst surface during the reaction it is of interest to study how the structure of the catalyst surface affects the reactivity with di-hydrogen as well as with adsorbed hydrogen atoms. In the present study we use a combination of experimental and theoretical methods to gain insight into how the structure of a cobalt surface affects the H2 dissociation reaction and the adsorption bond strength of the hydrogen atoms produced in this step. A comparison of the open Co(11–20) and (10–12) surfaces with the flat, close packed Co(0001) surface confirms that undercoordinated Co atoms strongly enhance the rate of H2 dissociation. At the same time, the lower desorption temperatures found on the more open surfaces indicate that the bond strength of adsorbed hydrogen decreases, in the following order: Co(0001)>Co(10–12)>Co(11–20). DFT calculations confirm this trend, showing that hydrogen adsorbs weaker on the more open surfaces for both low and high coverages. In the context of the Fischer-Tropsch synthesis reaction we propose that step and kink sites are important for efficient H2 dissociation. After dissociation, the higher hydrogen adsorption strength on terrace sites would promote diffusion away from the dissociation site to flat terraces where they can participate in hydrogenation reactions.
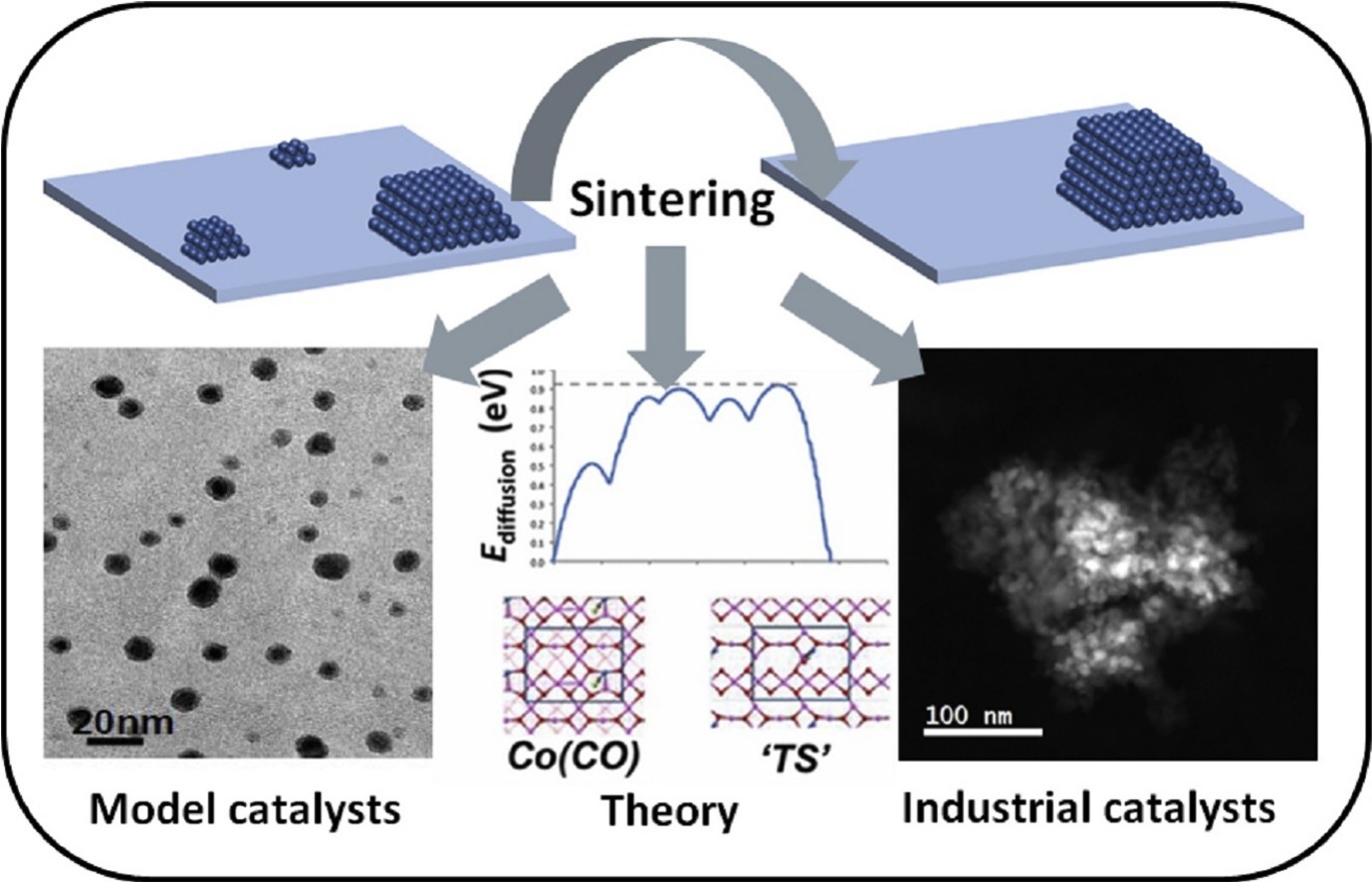
“Sintering of cobalt during FTS: Insights from industrial and model systems”
D. Moodley, M. Claeys, E. van Steen, P. van Helden, D. Kistamurthy, C.J. (Kees-Jan) Weststrate, J.W. (Hans) Niemantsverdriet, A. Saib, W. Erasmus, J. van de Loosdrecht, Catalysis Today 342 (2020) 59-70
Abstract
Sintering of supported cobalt catalysts is an important deactivation mechanism at play during realistic Fischer-Tropsch synthesis conditions. The traditional ways of studying the mechanism of sintering by looking at cobalt particle size distributions derived from transmission electron microscope images on porous supported catalysts, does have its drawbacks. In this mini-review, we show collaborative work between Sasol and its academic partners at the University of Cape Town and Eindhoven University of Technology, in which a two-pronged approach was used to study the topic of sintering. This included the use of model systems (model catalysts and theory) as well as in-situ monitoring of real (industrial) systems for understanding this practical problem in industry. We also show with new data, that the understanding generated, can be used in the mitigation of sintering by using either process conditions, catalyst design or catalyst regeneration.
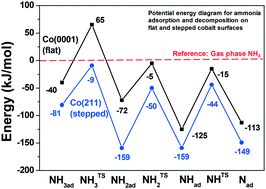
“Effect of ammonia on cobalt Fischer–Tropsch synthesis catalysts: a surface science approach”
A.C. (Ali Can) Kizilkaya, J.W. (Hans) Niemantsverdriet, and C.J. (Kees-Jan) Weststrate, Cat. Surf. & Tech 9 (2019) 702-710
Abstract
Ammonia adsorption and decomposition on defect-rich hcp-Co(0001) surfaces were investigated under ultra-high vacuum conditions in order to provide a fundamental explanation for industrially observed ammonia poisoning of cobalt based Fischer–Tropsch synthesis (FTS) catalysts. Temperature-programmed desorption, infrared spectroscopy and work function measurements indicate that undercoordinated sites bind ammonia stronger than sites on flat Co(0001), and they also induce its dehydrogenation. Density functional theory calculations were employed to explore the reactivity of defective Co surfaces using the fcc-Co(211) as a model. The results indicate that the decomposition products (NHx) adsorb strongly on or around the step site on fcc-Co(211). We find that NH (+2Had), adsorbed in the threefold site on the upper terrace, is equally stable as NH2 (+Had), adsorbed in the bridge position at the step edge, both being significantly more stable than the equivalent species adsorbed on the flat Co(0001). The calculated activation barriers for NH3,ad dehydrogenation steps are in reasonable agreement with the barriers obtained by fitting experimental data. Based on these fundamental insights, poisoning of cobalt nanoparticles during FTS by NH3 contaminants can be linked mainly to the blocking of undercoordinated sites by strongly adsorbed NH2 species.
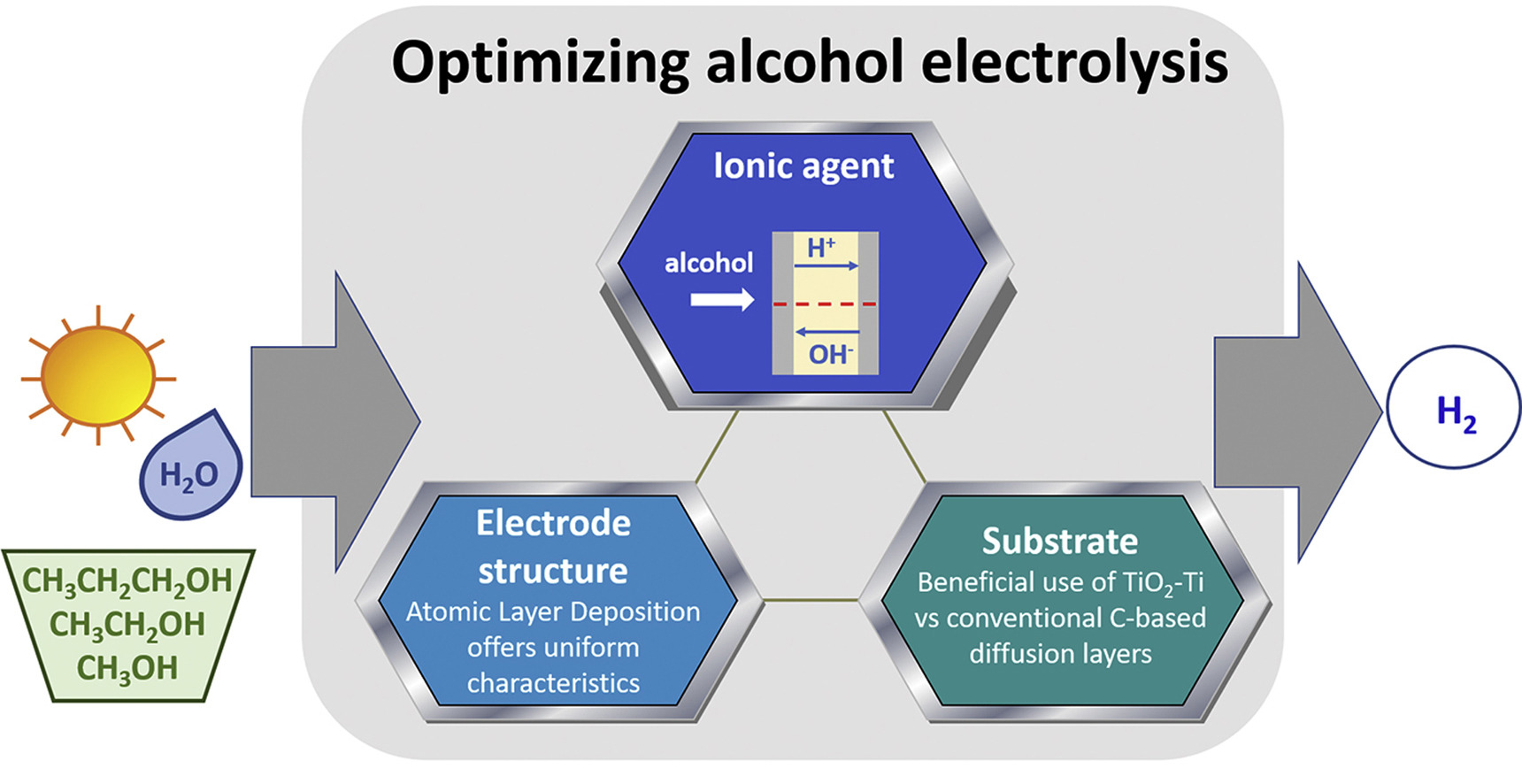
“Overpotential analysis of alkaline and acidic alcohol electrolysers and optimized membrane-electrode assemblies”
F.M. Sapountzi, V. Di Palma, G. Zafeiropoulos, H. Penchev, M.A. Verheijen, M. Creatore, F. Ublekov, V. Sinigersky, W.M. Arnold Bik, H.O.A. Fredriksson, M.N. Tsampas, J.W. (Hans) Niemantsverdriet, International Journal of Hydrogen Energy 44 (2019) 10163-10173
Abstract
Alcohol electrolysis using polymeric membrane electrolytes is a promising route for storing excess renewable energy in hydrogen, alternative to the thermodynamically limited water electrolysis. By properly choosing the ionic agent (i.e. H+ or OH−) and the catalyst support, and by tuning the catalyst structure, we developed membrane-electrode-assemblies which are suitable for cost-effective and efficient alcohol electrolysis. Novel porous electrodes were prepared by Atomic Layer Deposition (ALD) of Pt on a TiO2-Ti web of microfibers and were interfaced to polymeric membranes with either H+ or OH− conductivity. Our results suggest that alcohol electrolysis is more efficient using OH− conducting membranes under appropriate operation conditions (high pH in anolyte solution). ALD enables better catalyst utilization while it appears that the TiO2-Ti substrate is an ideal alternative to the conventional carbon-based diffusion layers, due to its open structure. Overall, by using our developmental anodes instead of commercial porous electrodes, the performance of the alcohol electrolyser (normalized per mass of Pt) can be increased up to ∼30 times.
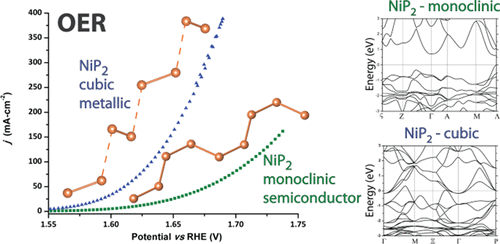
“NiP2: A Story of Two Divergent Polymorphic Multifunctional Materials”
J. Xu, D.Y. Petrovyk, O. Bondarchuk, Y. Ziouani, N. González-Ballesteros, P. Yox, F.M. Sapountzi, J.W. (Hans) Niemantsverdriet, Y.V. Kolen’ko, K. Kovnir, Chem. Mater. 31 (2019) 3407-3418
Abstract
In this paper, we explore the drastic differences in transport properties and catalytic activities for two structural polymorphs of NiP2: cubic (Pa3; No. 205) and monoclinic NiP2 (C2/c; No. 15). The former one has been long assumed to be the high-pressure metastable phase, as it had been originally synthesized through high-pressure methods. Synthetic and in situ synchrotron X-ray diffraction studies unambiguously show that the cubic polymorph can be synthesized at ambient pressure but irreversibly transforms into the monoclinic structure above 876 K. Band structure calculations and transport measurements show that cubic NiP2 is a semimetal, while the monoclinic polymorph is an n-type direct band gap semiconductor. Both compounds exhibit low thermal conductivities, with cubic NiP2 exhibiting a value of 1.7 W•m–1 K–1 at 300 K. The bulk structure of the phosphides may affect the surface-related properties. Unlike the monoclinic polymorph, cubic NiP2 excels in both half-cell HER and OER measurements. In alkaline half-cell OER, cubic NiP2 outperforms the RuO2 standard. More importantly, HER tests in a PEM electrolysis single cell showed high promise for cubic NiP2, which requires only 13% higher overpotentials when compared to state-of-the-art Pt/IrRuOx-based assemblies, far surpassing any reported properties of metal pnictide or chalcogenide full cells.
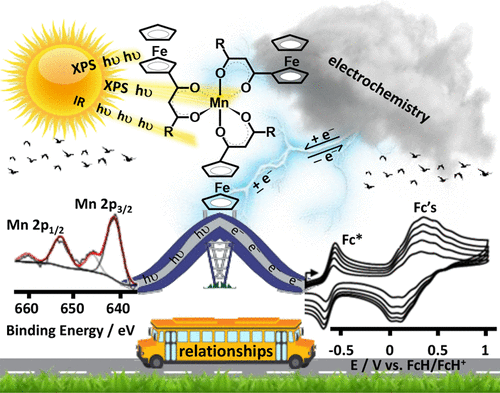
“Can Electrochemical Measurements Be Used To Predict X-ray Photoelectron Spectroscopic Data? The Case of Ferrocenyl-β-Diketonato Complexes of Manganese(III)”
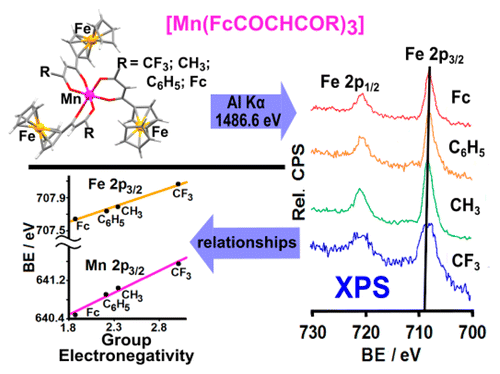
B.E. Buitendach, E. Erasmus, J.W. (Hans) Niemantsverdriet, and J.C. Swarts, Inorg. Chem. 57 (2018) 6606-6616
Abstract
In order to better understand intramolecular communication between molecular fragments, a series of ferrocene-functionalized β-diketonato manganese(III) complexes, [Mn(FcCOCHCOR)3] with R = CF3, 1, CH3, 2, Ph = C6H5, 3, and Fc = FeII(η5-C5H4)(η5-C5H5), 4, the mixed ligand β-diketonato complex [Mn(FcCOCHCOFc)2(FcCOCHCOCH3)], 5, as well as the acac complex [Mn(CH3COCHCOCH3)3], 6, were subjected to an electrochemical and X-ray photoelectron spectroscopy (XPS) study. The ferrocenyl (FeII) and MnIII redox potentials, E°′, and photoelectron lines were sufficiently resolved in each complex to demonstrate a linear correlation between E°′ and group electronegativities of ligand R groups, χR, or ΣχR, as well as with binding energies of both the Fe 2p3/2 and Mn 2p3/2 photoelectron lines. These relationships are consistent with effective communication between molecular fragments of 1–5. From these relationships, prediction of Mn and Fe core electron binding energies in [Mn(R1COCHCOR2)3] complexes from known manganese and/or ferrocenyl redox potentials are, therefore, now possible. Ligand infrared carbonyl stretching frequencies were successfully related to binding energy as a measure of the energy required for inner-sphere reorganization. In particular it became possible to explain why, upon electrochemical oxidation or photoionization, the ferrocenyl FeII inner-shell of 1–5 needs more energy in complexes with ligands bearing electron-withdrawing (CF3) groups than in ligands bearing electron-donating groups such as ferrocenyl. The XPS determined entity Iratio (the ratio between the intensities of the satellite and main metal 2p3/2 photoelectron lines) is an indication not only of the amount of charge transferred, but also of the degree of inner-sphere reorganization. Just as for binding energy, the quantity Iratio was also found to be related to the energy requirements for the inner-sphere reorganization depicted by the vibrational frequency, vco.
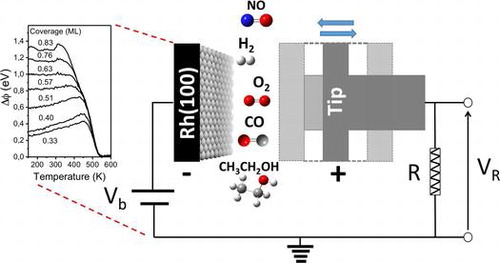
“Application of work function measurements in the study of surface catalyzed reactions on Rh(100)”
B. Caglar, A.C. Kizilkaya, J.W. (Hans) Niemantsverdriet, and C.J. (Kees-Jan) Weststrate, Catalysis Structure & Reactivity 4 (2018) 1-11
Abstract
The present article aims to show how work function measurements (WF) can be applied in the study of elementary surface reaction steps on metallic single crystal surfaces. The work function itself can in many cases not be interpreted directly, as it lacks direct information on structural and chemical nature of the surface and adsorbates, but it can be a powerful tool when used together with other surface science techniques which provide information on the chemical nature of the adsorbed species. We here, illustrate the usefulness of work function measurements using Rh(100) as our model catalyst. The examples presented include work function measurements during adsorption, surface reaction, and desorption of a variety of molecules relevant for heterogeneous catalysis. Surface coverage of adsorbates, isosteric heat of adsorption, and kinetic parameters for desorption, desorption/decomposition temperatures of surface species, different reaction regimes were determined by WF with the aid of other surface science techniques.
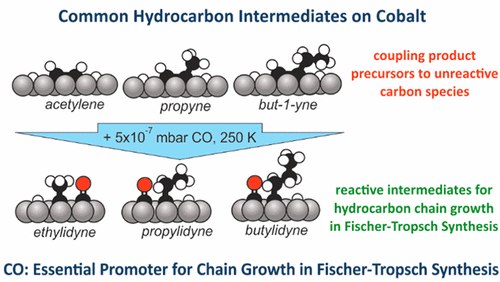
“CO as a Promoting Spectator Species of CxHy Conversions Relevant for Fischer–Tropsch Chain Growth on Cobalt: Evidence from Temperature-Programmed Reaction and Reflection Absorption Infrared Spectroscopy”
C.J. Weststrate, J.W. Niemantsverdriet, ACS Catal. 8 (2018) 10826-10835
Abstract
Cobalt-catalyzed low temperature Fischer–Tropsch synthesis is a prime example of an industrially relevant reaction in which CxHy intermediates involved in chain growth react in the presence of a large quantity of COad. In this study, we use a Co(0001) single-crystal model catalyst to investigate how CO, adsorbed alongside CxHy adsorbates affects their reactivity. Temperature-programmed reaction spectroscopy was used to determine the hydrogen content of the CxHy intermediates formed at different temperatures, and infrared absorption spectroscopy was used to obtain more specific information on the chemical identity of the various reaction intermediates formed. Ethene, propene, and but-1-ene precursors decompose below 200 K. The 1-alkyne adsorbate is identified as a major product, and some alkylidyne species form as well when the initial alkene coverage is high. The surface hydrogen atoms produced in the low temperature decomposition step start leaving the surface >300 K. When an alkyne/Had-covered surface is heated in the presence of CO, the alkyne adsorbates are hydrogenated to the corresponding alkylidyne at temperatures <250 K. This finding shows that CxHy surface species react differently in the presence of COad, a notion of general importance for catalytic reactions where both CO and CxHy species are present. In the context of Fischer–Tropsch synthesis, the observed CO-induced reaction is of specific importance for the alkylidyne chain growth mechanism. In this reaction, scheme hydrocarbon chains grow via coupling of CHad with a (Cn) alkylidyne adsorbate to produce the (Cn+1) alkyne. A subsequent hydrogenation of the alkyne product to the corresponding alkylidyne is required for further growth. The present work shows that this specific reaction is promoted by the presence of CO. This suggests that the influence of CO spectators on the stability of CxHy surface intermediates is beneficial for efficient chain growth.
“Iron Carbidization on Thin-Film Silica and Silicon: A Near-Ambient-Pressure X-ray Photoelectron Spectroscopy and Scanning Tunneling Microscopy Study”
X. Zhou, G.J.A. Mannie, J. Yin, X. Yu, C.J. Weststrate, X. Wen, K. Wu, Y. Yang, Y. (Yongwang) Li, J.W. (Hans) Niemantsverdriet, ACS Catal. 8 (2018) 7326-7333
Abstract
Model catalysts consisting of iron particles with similar size deposited on thin-film silica (Fe/SiO2) and on silicon (Fe/Si) were used to study iron carbidization in a CO atmosphere using in situ near-ambient-pressure X-ray photoelectron spectroscopy. Significant differences were observed for CO adsorption, CO dissociation, and iron carbidization when the support was changed from thin-film silica to silicon. Stronger adsorption of CO on Fe/Si than that on Fe/SiO2 was evident from the higher CO equilibrium coverage found at a given temperature in the presence of 1 mbar of CO gas. On thin-film silica, iron starts to carbidize at 150 °C, while the onset of carbidization is at 100 °C on the silicon support. The main reason for the different onset temperature for carbidization is the efficiency of removal of oxygen species after CO dissociation. On thin-film silica, oxygen species formed by CO dissociation block the iron surface until ∼150 °C, when CO2 formation removes surface oxygen. Instead, on the silicon support, oxygen species readily spill over to the silicon. As a consequence, oxygen removal is not rate-limiting anymore and carbidization of iron can proceed at a lower temperature.
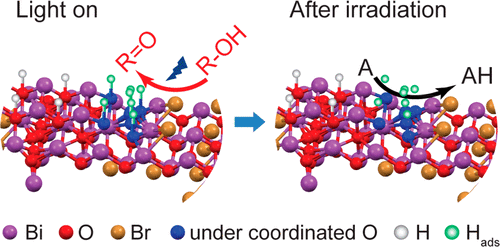
“Efficient Solar-Driven Hydrogen Transfer by Bismuth-Based Photocatalyst with Engineered Basic Sites”
Y. Dai, C. Li, Y. Shen, S. Zhu, M.S. Hvid, L. Wu, J. Skibsted, Y. (Yongwang) Li, J.W. (Hans) Niemantsverdriet, F. Besenbacher, N. Lock, R. Su, JACS 140 (2018) 16711-16719
Abstract
Photocatalytic organic conversions involving a hydrogen transfer (HT) step have attracted much attention, but the efficiency and selectivity under visible light irradiation still needs to be significantly enhanced. Here we have developed a noble metal-free, basic-site engineered bismuth oxybromide [Bi24O31Br10(OH)δ] that can accelerate the photocatalytic HT step in both reduction and oxidation reactions, i.e., nitrobenzene to azo/azoxybenzene, quinones to quinols, thiones to thiols, and alcohols to ketones under visible light irradiation and ambient conditions. Remarkably, quantum efficiencies of 42% and 32% for the nitrobenzene reduction can be reached under 410 and 450 nm irradiation, respectively. The Bi24O31Br10(OH)δ photocatalyst also exhibits excellent performance in up-scaling and stability under visible light and even solar irradiation, revealing economic potential for industrial applications.
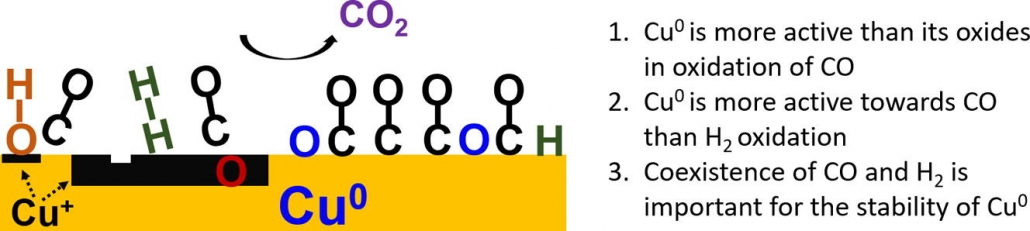
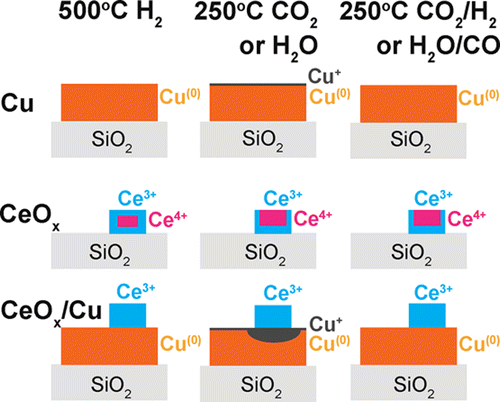
“Preferential oxidation of CO in H2 on Cu and Cu/CeOx catalysts studied by in situ UV–Vis and mass spectrometry and DFT”
Y. Bu, S. Er, J.W. (Hans) Niemantsverdriet, H.O.A. Fredriksson, J. Catal. 357 (2018) 176-187
Abstract
Preferential oxidation of CO in H2 was studied by in situ ultraviolet–visible (UV–Vis) and mass spectrometry on flat model Cu and Cu/CeOx catalysts. The experimental findings were interpreted and compared with the results from density functional theory (DFT) calculations of the adsorption and activation energies for the essential reaction steps on Cu(1 1 1). It was found that oxidation of CO preferentially takes place on Cu(0) and that no significant H2 oxidation took place under any of the investigated conditions. The presence of CeOx accelerates Cu(0)-oxidation which leads to catalyst deactivation. In contrast, CeOx promotes the CO oxidation rate on catalysts that were already oxidized to CuOx. The coexistence of CO and H2 is important to sustain the stability of metallic Cu and thereby a high rate of CO2 formation. In pure CO/O2 gas, the metallic phase can only be maintained as long as full O2 conversion is reached. In pure H2/O2, Cu is always partly but never fully oxidized, suggesting that a passivating surface layer is formed. This is also the case for H2 rich gas mixtures with small amounts of CO and O2. The most active surface termination, Cu(0), can therefore not be maintained under the industrially most interesting reaction condition where full conversion of trace amounts of CO in H2 is required. DFT calculations predict that the dissociative H2 adsorption is a key limiting step for hydrogen oxidation on the Cu(1 1 1) surface, especially when the low sticking coefficient is taken into account.
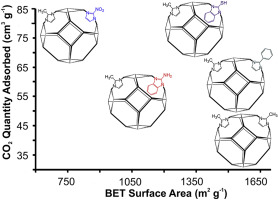
“Enhanced CO2 adsorption in nano-ZIF-8 modified by solvent assisted ligand exchange”
C. Tsai, J.W. (Hans) Niemantsverdriet, E.H.G. Langner, Microporous and Mesoporous Materials 262 (2018) 98-105
Abstract
The organic linkers of Zeolitic Imidazole Framework-8 (ZIF-8) nanoparticles influence its adsorption of CO2 gases. Solvent Assisted Ligand Exchange (SALE) was successfully used to exchange ∼ 13% of the 2-methylimidazolate linkers of ZIF-8 nanoparticles with 2-mercaptobenzimidazole, 2-aminobenzimidazole or 2-phenylimidazole. With 2-nitroimidiazole ∼67% exchange was achieved, since no bulky benzyl groups were present. During SALE treatment the SOD (sodalite zeolitic framework type) topology of ZIF-8 was maintained, but after the higher exchange percentage of 2-nitroimidazole an frl topology was observed. Up to a two-fold increase in CO2 adsorption was recorded after SALE of ZIF-8 with imidazole derivatives containing NO2 and SH electron withdrawing functional groups. In the case of 2-aminobenzimidazole only a moderate increase in CO2 adsorption was observed.
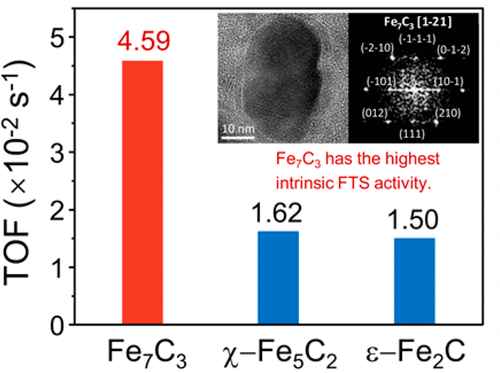
“Relationship between Iron Carbide Phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and Catalytic Performances of Fe/SiO2 Fischer–Tropsch Catalysts”
Q. Chang, C. Zhang, C. Liu, Y. Wei, A.V. Cheruvathur, A.I. Dugulan, J.W. (Hans) Niemantsverdriet, X. Liu, Y. He, M. Qing, L. Zheng, Y. Yun, Y. Yang. Y. (Yongwang) Li, ACS Catal. 8 (2018) 3304-3316
Abstract
The influence of different iron carbides on the activity and selectivity of iron-based Fischer–Tropsch catalysts has been studied. Different iron carbide phases are obtained by the pretreatment of a binary Fe/SiO2 model catalyst (prepared by coprecipitation method) to different gas atmospheres (syngas, CO, or H2). The phase structures, compositions, and particle sizes of the catalysts are characterized systematically by XRD, XAFS, MES, and TEM. It is found that in the syngas-treated catalyst only χ-Fe5C2 carbide is formed. In the CO-treated catalyst, Fe7C3 and χ-Fe5C2 with a bimodal particle size distribution are formed, while the H2-treated catalyst exhibits the bimodal size distributed ε-Fe2C and χ-Fe5C2 after a Fischer–Tropsch synthesis (FTS) reaction. The intrinsic FTS activity is calculated and assigned to each corresponding iron carbide based on the phase composition and the particle size. It is identified that Fe7C3 has the highest intrinsic activity (TOF = 4.59 × 10–2 s–1) among the three candidate carbides (ε-Fe2C, Fe7C3, and χ-Fe5C2) in typical medium-temperature Fischer–Tropsch (MTFT) conditions (260–300 °C, 2–3 MPa, and H2/CO = 2). Moreover, FTS over ε-Fe2C leads to the lowest methane selectivity.
“Light-tuned selective photosynthesis of azo- and azoxy-aromatics using graphitic C3N4“
Yitao Dai, Chao Li, Yanbin Shen, Jian Xu, Hans Niemantsverdriet, Yongwang Li, Flemming Besenbacher, Nina Lock, Ren Su, Nature Communications 9 (2018) Article number 60
(Open access)
Abstract
Solar-driven photocatalysis has attracted significant attention in water splitting, CO2 reduction and organic synthesis. The syntheses of valuable azo- and azoxyaromatic dyes via selective photoreduction of nitroaromatic compounds have been realised using supported plasmonic metal nanoparticles at elevated temperatures (≥90 °C); however, the high cost, low efficiency and poor selectivity of such catalyst systems at room temperature limit their application. Here we demonstrate that the inexpensive graphitic C3N4 is an efficient photocatalyst for selective syntheses of a series of azo- and azoxy-aromatic compounds from their corresponding nitroaromatics under either purple (410 nm) or blue light (450 nm) excitation. The high efficiency and high selectivity towards azo- and azoxy-aromatic compounds can be attributed to the weakly bound photogenerated surface adsorbed H-atoms and a favourable N-N coupling reaction. The results reveal financial and environmental potential of photocatalysis for mass production of valuable chemicals.
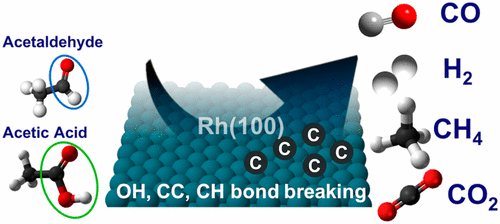
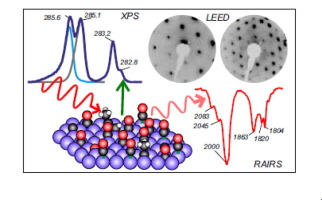
“Effect of Aldehyde and Carboxyl Functionalities on the Surface Chemistry of Biomass-Derived Molecules”
B. Caglar, J.W. (Hans) Niemantsverdriet, C.J. (Kees-Jan) Weststrate, Langmuir 33 (2017) 11919-11929
Abstract
The adsorption and decomposition of acetaldehyde and acetic acid were studied on Rh(100) to gain insight into the interaction of aldehyde and carboxyl groups of biomass-derived molecules with the surface. Temperature-programmed reaction spectroscopy (TPRS) was used to monitor gaseous reaction products, whereas Reflection absorption infrared spectroscopy (RAIRS) was used to determine the nature of surface intermediates and reaction paths. The role of adsorbate interactions in oxygenate decomposition chemistry was also investigated by varying the surface coverage. Acetaldehyde adsorbs in an η2(C, O) configuration for all coverages, where the carbonyl group binds to the surface via the C and O atoms. Decomposition occurs below room temperature (180–280 K) via C–H and C–C bond breaking, which releases CO, H, and CHx species on the surface. At low coverage, CHx dehydrogenation dominates and surface carbon is produced alongside H2 and CO. At high coverage, about 60% of the CHx hydrogenates to form methane, whereas only 40% of the CHx decomposes further to surface carbon. Acetic acid adsorbs dissociatively on the Rh(100) surface via O–H bond scission, forming a mixture of mono- and bidentate acetate. The decomposition of acetate proceeds via two different pathways: (i) deoxygenation via C–O and C–C bond scissions and (ii) decarboxylation via C–C bond scission. At low coverage, the decarboxylation pathway dominates, a process that occurs at slightly above room temperature (280–360 K) and produces CO2 and CHx, where the latter decomposes further to surface carbon and H2. At high coverage, both decarboxylation and deoxygenation occur, slightly, above room temperature (280–360 K). The resulting O adatoms produced in the deoxygenation path react with surface hydrogen or CO to form water and CO2, respectively. The CHx species dehydrogenate to surface carbon for all coverages. Our findings suggest that oxygenates with a C═O functionality and an alkyl end react on the Rh(100) surface to produce synthesis gas and small hydrocarbons whereas CO2 and synthesis gas are produced when oxygenates with a COOH functionality and an alkyl end react with the Rh(100) surface. For both cases, carbon accumulation occurs on the surface.
“Understanding FTS selectivity: the crucial role of surface hydrogen”
C. J. Weststrate and J. W. Niemantsverdriet, Faraday Discuss. 197 (2017) 101-116
Abstract
Monomeric forms of carbon play a central role in the synthesis of long chain hydrocarbons via the Fischer–Tropsch synthesis (FTS). We explored the chemistry of C1Hxad species on the close-packed surface of cobalt. Our findings on this simple model catalyst highlight the important role of surface hydrogen and vacant sites for product selectivity. We furthermore find that COad affects hydrogen in multiple ways. It limits the adsorption capacity for Had, lowers its adsorption energy and inhibits dissociative H2 adsorption. We discuss how these findings, extrapolated to pressures and temperatures used in applied FTS, can provide insights into the correlation between partial pressure of reactants and product selectivity. By combining the C1Hx stability differences found in the present work with literature reports of the reactivity of C1Hx species measured by steady state isotope transient kinetic analysis, we aim to shed light on the nature of the atomic carbon reservoir found in these studies.
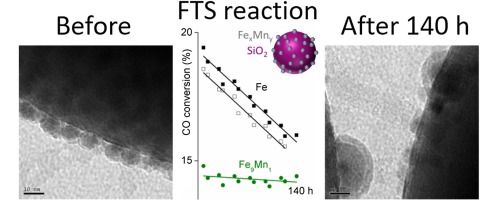
“SiO2-supported Fe & FeMn colloids—Fischer-Tropsch synthesis on 3D model catalysts”
Mohammadhassan Dad, Remco J. Lancee, Matthys Janse van Vuuren, Jan van de Loosdrecht, J.W. (Hans) Niemantsverdriet, Hans O.A. Fredriksson, Appl. Catal. A 537 (2017) 83–92
Abstract
A well-defined model catalyst constituting a compromise between high surface area, porous, industrial catalysts and a planar model catalyst has been developed. It allows for measurements of catalytic activity in micro reactors, where diffusion problems are kept at a minimum, while characterization both by surface science techniques and by bulk techniques can be applied. Monodisperse, non-porous SiO2 microspheres with diameter 875 ± 25 nm have been synthesized, serving as the large area model support. These where then impregnated with pre-formed, monodisperse, colloidal Fe and FeMn nanoparticles resulting in a three-dimensional equivalent of a flat, Fe(-Mn)/SiO2 model catalysts. Characterization with electron microscopy (SEM and TEM), X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD), before and after catalytic testing was performed. It was shown that the model catalysts can be used in Fischer-Tropsch synthesis experiments under industrially relevant conditions. The FTS experiments reveal that compared to the pure Fe catalyst, FeMn shows more stable activity, higher selectivity towards olefins and lower selectivity toward CH4 and CO2. Significant amounts of hydrocarbons on the catalyst surfaces and some minor indications of sintering were detected after the reaction. Formation of FeCx was detected for the Fe catalyst while no significant amounts could be seen on the Mn-promoted catalyst.
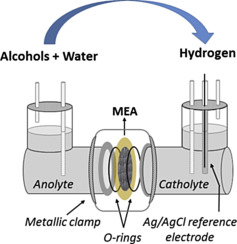
“Hydrogen from electrochemical reforming of C1–C3 alcohols using proton conducting membranes”
F.M. Sapountzi, M.N. Tsampas, H.O.A. Fredriksson, J.M. Gracia, J.W. Niemantsverdriet, International Journal of Hydrogen Energy 42 (2017) 10762–10774
Abstract
This study investigates the production of hydrogen from the electrochemical reforming of short-chain alcohols (methanol, ethanol, iso-propanol) and their mixtures. High surface gas diffusion Pt/C electrodes were interfaced to a Nafion polymeric membrane. The assembly separated the two chambers of an electrochemical reactor, which were filled with anolyte (alcohol + H2O or alcohol + H2SO4) and catholyte (H2SO4) aqueous solutions. The half-reactions, which take place upon polarization, are the alcohol electrooxidation and the hydrogen evolution reaction at the anode and cathode, respectively. A standard Ag/AgCl reference electrode was introduced for monitoring the individual anodic and cathodic overpotentials. Our results show that roughly 75% of the total potential losses are due to sluggish kinetics of the alcohol electrooxidation reaction. Anodic overpotential becomes larger as the number of C-atoms in the alcohol increases, while a slight dependence on the pH was observed upon changing the acidity of the anolyte solution. In the case of alcohol mixtures, it is the largest alcohol that dictates the overall cell performance.
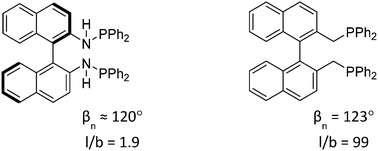
“Ligand effects in rhodium-catalyzed hydroformylation with bisphosphines: steric or electronic?”
Yunzhe Jiao, Marta Serrano Torne, Jose Gracia, J. W. (Hans) Niemantsverdriet and Piet W. N. M. van Leeuwen, Catal. Sci. Technol. 7 (2017) 1404-1414
Abstract
Twelve commercially available bisphosphine ligands have been evaluated in rhodium-catalyzed hydroformylation reactions. All ligands exhibited high chemoselectivities for aldehyde formation. The highest enantioselectivity (53% ee) of styrene hydroformylation was achieved with (S)-BTFM-Garphos (L7) substituted with electron withdrawing substituents. High pressure NMR (HP-NMR) spectroscopy and in situ high pressure IR spectroscopy (HP-IR) were used to study the resting states of the catalyst species in the reactions. The ligand effect on the structures of the observable species was examined. Both electronic and steric factors were considered to contribute to the performance of the various ligands. The results showed that decreasing the phosphine basicity increased the enantioselectivity, while in the systems studied here the steric character plays a less important role than the electronic features in achieving good regioselectivities.
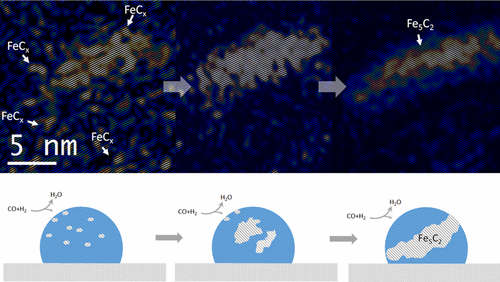
“Environmental Transmission Electron Microscopy (ETEM) Studies of Single Iron Nanoparticle Carburization in Synthesis Gas”
Xi Liu, Chenghua Zhang, Yongwang Li, J. W. Niemantsverdriet, Jakob B. Wagner, and Thomas W. Hansen, ACS Catal. 7 (2017) 4867−4875
Abstract
Structural evolution of iron nanoparticles involving the formation and growth of iron carbide nuclei in the iron nanoparticle was directly visualized at the atomic level, using environmental transmission electron microscopy (TEM) under reactive conditions mimicking Fischer−Tropsch synthesis. Formation of the iron carbide nuclei and surface reconstruction of the iron nanoparticle play an essential role in carburization of the iron nanoparticle and consequent formation of Fe5C2. Identification of carbide and oxide intermediates evidenced by high-resolution TEM images, electron diffraction patterns and electron energy-loss spectra provides a detailed picture from initial activation to final degradation of iron under synthesis gas.
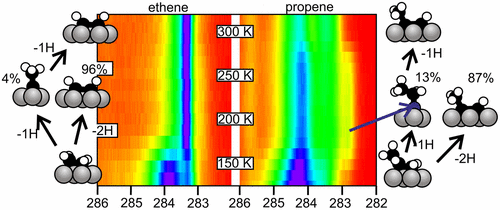
“Adsorption and Decomposition of Ethene and Propene on Co(0001): The Surface Chemistry of Fischer–Tropsch Chain Growth Intermediates”
C. J. Weststrate, Ionel M. Ciobîcă, Jan van de Loosdrecht, and J. W. Niemantsverdriet, J. Phys. Chem. C 120 (2016) 29210–29224
Abstract
Experiments that provide insight into the elementary reaction steps of CxHy adsorbates are of crucial importance to better understand the chemistry of chain growth in Fischer–Tropsch synthesis (FTS). In the present study we use a combination of experimental and theoretical tools to explore the reactivity of C2Hx and C3Hx adsorbates derived from ethene and propene on the close-packed surface of cobalt. Adsorption studies show that both alkenes adsorb with a high sticking coefficient. Surface hydrogen does not affect the sticking coefficient but reduces the adsorption capacity of both ethene and propene by 50% and suppresses decomposition. On the other hand, even subsaturation quantities of COad strongly suppress alkene adsorption. Partial alkene dehydrogenation occurs at low surface temperature and predominantly yields acetylene and propyne. Ethylidyne and propylidyne can be formed as well, but only when the adsorbate coverage is high. Translated to FTS, the stable, hydrogen-lean adsorbates such as alkynes and alkylidynes will have long residence times on the surface and are therefore feasible intermediates for chain growth. The comparatively lower desorption barrier for propene relative to ethene can to a large extent be attributed to the higher stability of the molecule in the gas phase, where hyperconjugation of the double bond with σ bonds in the adjacent methyl group provides additional stability to propene. The higher desorption barrier for ethene can potentially contribute to the anomalously low C2Hx production rate that is typically observed in cobalt-catalyzed FTS.
“Role of ZnO and CeOx in Cu-Based Model Catalysts in Activation of H2O and CO2 Dynamics Studied by in Situ Ultraviolet–Visible and X-ray Photoelectron Spectroscopy”
Yibin Bu, C. J. Weststrate, J. W. Niemantsverdriet, and Hans O. A. Fredriksson, ACS Catal. 6 (2016) 7994–8003
Abstract
Flat model and powder Cu, ZnO/Cu, and CeOx/Cu catalysts were studied by focusing on the role of the oxide phase as a promoter in the water gas shift (WGS) and its reverse reaction (RWGS). Activity measurements of the powder catalysts showed that both oxides enhance Cu reactivity, with CeOx/Cu being more active than ZnO/Cu in the WGS reaction. In situ ultraviolet–visible spectroscopy, exploiting the localized surface plasmon resonances of metallic Cu nanoparticles, together with X-ray photoelectron spectroscopy was then used to elucidate the origin of the enhanced reactivity on flat model catalysts. These experiments showed that ZnO and CeOx promote H2O and CO2 dissociation, leading to oxidation of the Cu nanoparticles. CeOx performs better in this respect than ZnO. This is important because the reactivity in the WGS and RWGS reactions is related to the ability to activate H2O and CO2. The Ce3+ ions are identified as the most efficient sites for H2O and CO2 dissociation, while Cu0 keeps Ce3+ stable by promoting reduction of Ce4+ during the dissociation process. In this sense, the CeOx/Cu catalyst forms a bifunctional catalyst, which is more active in the (R)WGS than CeOx and Cu catalysts separately.
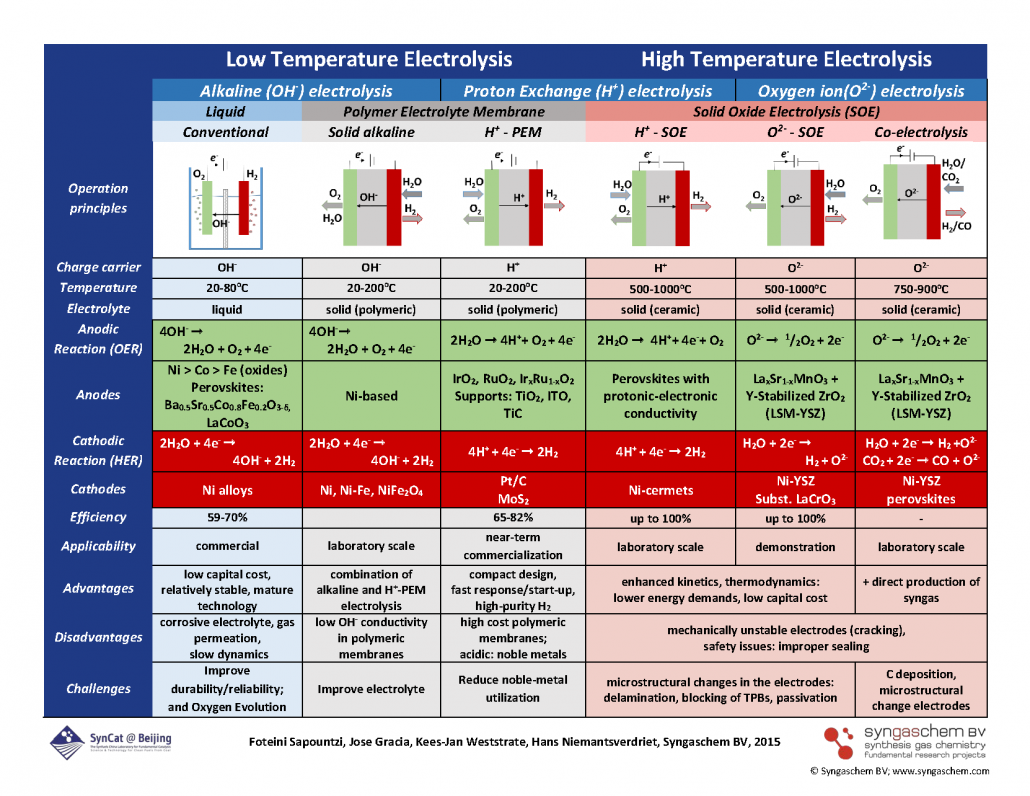
“Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas“
Foteini M. Sapountzi, Jose M. Gracia, C.J. (Kees-Jan) Weststrate, Hans O.A. Fredriksson, J.W. (Hans) Niemantsverdriet, Progress in Energy and Combustion Science 58 (2017) 1–35
Abstract
Water electrolysis is the most promising method for efficient production of high purity hydrogen (and oxygen), while the required power input for the electrolysis process can be provided by renewable sources (e.g. solar or wind). The thus produced hydrogen can be used either directly as a fuel or as a reducing agent in chemical processes, such as in Fischer–Tropsch synthesis. Water splitting can be realized both at low temperatures (typically below 100 °C) and at high temperatures (steam water electrolysis at 500–1000 °C), while different ionic agents can be electrochemically transferred during the electrolysis process (OH−, H+, O2−). Singular requirements apply in each of the electrolysis technologies (alkaline, polymer electrolyte membrane and solid oxide electrolysis) for ensuring high electrocatalytic activity and long-term stability. The aim of the present article is to provide a brief overview on the effect of the nature and structure of the catalyst–electrode materials on the electrolyzer’s performance. Past findings and recent progress in the development of efficient anode and cathode materials appropriate for large-scale water electrolysis are presented. The current trends, limitations and perspectives for future developments are summarized for the diverse electrolysis technologies of water splitting, while the case of CO2/H2O co-electrolysis (for synthesis gas production) is also discussed.
“Spectroscopic insights into cobalt-catalyzed Fischer-Tropsch synthesis: A review of the carbon monoxide interaction with single crystalline surfaces of cobalt“
C.J. Weststrate, J. van de Loosdrecht, J.W. Niemantsverdriet, J. Catal. 342 (2016) 1
Abstract
The present article summarizes experimental findings of the interaction of CO with single crystal surfaces of cobalt. We first provide a quantitative study of non-dissociative CO adsorption on Co(0001) and establish a quantitative correlation between θCO and adsorption site occupation. In light of these findings we revisit the structure of previously reported ordered CO/Co(0001) adsorbate layers. Measurements of the CO coverage at equilibrium conditions are used to derive a phase diagram for CO on Co(0001). For low temperature Fischer-Tropsch synthesis conditions the CO coverage is predicted to be ≈0.5 ML, a value that hardly changes with pCO. The CO desorption temperature found in temperature programmed desorption is practically structure-independent, despite structure-dependent heats of adsorption reported in the literature. This mismatch is attributed to a structure-dependent pre-exponential factor for desorption. IR spectra reported throughout this study provide a reference point for IR studies on cobalt catalysts. Results for CO adsorbed on flat and defect-rich Co surfaces as well as particular, CO adsorbed on top sites, and in addition affect the distribution of COad over the various possible adsorption sites.
“Reflections on the Fischer-Tropsch synthesis: Mechanistic issues from a surface science perspective”
C.J. Weststrate, P. van Helden, J.W. (Hans) Niemantsverdriet, Catalysis Today 275 (2016) 100
Abstract
The current paper presents a mechanistic view on important steps in the Fischer-Tropsch synthesis on cobalt catalysts, inspired by surface science studies. By revisiting the relation between activity and selectivity that results from the ASF assumption we highlight that knowledge about the number of growing chains as well as their residence time (∼growth rate) is of crucial importance to sketch a physically realistic scenario for FTS. This motivates further investigations into the microscopic scenario for FTS chain growth on fcc cobalt nanoparticles, by looking into the reaction mechanism in relation to surface structure and by determining the activation energies for key elementary steps. Such studies indicate that the modest activity of Co FTS catalysts might very well be attributable to the difficulty to remove chemisorbed oxygen from the metallic surface, rather than to dissociation of CO, which was found to proceed readily at step edge sites. Chain growth is envisaged to take place on the close-packed surfaces, with chain initiation via CH + CH to form acetylene, followed by hydrogenation to form ethylidyne, C-CH3, a reaction that is shown to be promoted by co-adsorbed CO. Ethylidyne then couples with CH to form propyne, HC-C-CH3, etc. We propose that a fairly large number of surface sites is involved in the growth of a single chain. In such a “growth ensemble” multiple active step sites produce CHx monomer species that spill over onto the same close-packed coupling terrace, where one or only a few chains grow at the same time. In such a scenario diffusion of hydrocarbonaceous surface species is an essential step in the overall reaction sequence. We explore which factors need to be taken into account when considering of CxHy species under realistic reaction conditions. In addition, we note that the coupling reaction itself, via CH + C-CnH2n+1, is a source of growing chain mobility.
“Properties of Manganese(III) Ferrocenyl-β-Diketonato Complexes Revealed by Charge Transfer and Multiplet Splitting in the Mn 2p and Fe 2p X-Ray Photoelectron Envelopes“
Blenerhassitt E. Buitendach, Elizabeth Erasmus, J. W. (Hans) Niemantsverdriet, and Jannie C. Swarts, Molecules 21 (2016) 1427
Abstract
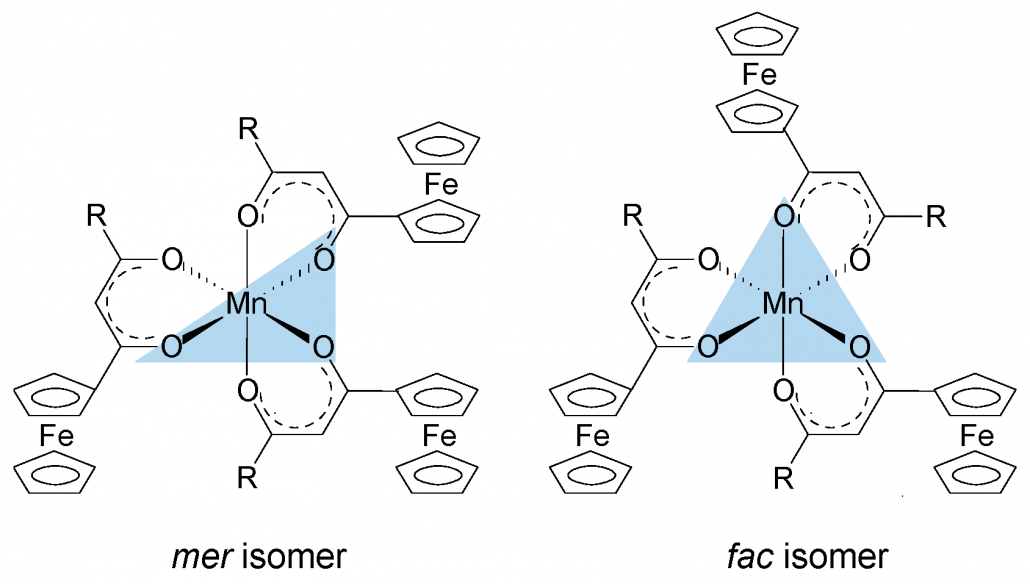
A series of ferrocenyl-functionalized β-diketonato manganese(III) complexes, [Mn(FcCOCHCOR)3] with R = CF3, CH3, Ph (phenyl) and Fc (ferrocenyl) was subjected to a systematic XPS study of the Mn 2p3/2 and Fe 2p3/2 core-level photoelectron lines and their satellite structures. A charge-transfer process from the β-diketonato ligand to the Mn(III) metal center is responsible for the prominent shake-up satellite peaks of the Mn 2p photoelectron lines and the shake-down satellite peaks of the Fe 2p photoelectron lines. Multiplet splitting simulations of the photoelectron lines of the Mn(III) center of [Mn(FcCOCHCOR)3] resemble the calculated Mn 2p3/2 envelope of Mn3+ ions well, indicating the Mn(III) centers are in the high spin state. XPS spectra of complexes with unsymmetrical β-diketonato ligands (i.e., R not Fc) were described with two sets of multiplet splitting peaks representing fac and the more stable mer isomers respectively. Stronger electron-donating ligands stabilize fac more than mer isomers. The sum of group electronegativities, ΣχR, of the β-diketonato pendant side groups influences the binding energies of the multiplet splitting and charge transfer peaks in both Mn and Fe 2p3/2 photoelectron lines, the ratio of satellite to main peak intensities, and the degree of covalence of the Mn–O bond.
“Consequences of Electron-Density Manipulations on the X-ray Photoelectron Spectroscopic Properties of Ferrocenyl-β-diketonato Complexes of Manganese(III). Structure of [Mn(FcCOCHCOCH3)3]”
Blenerhassitt E. Buitendach, Elizabeth Erasmus, Marilé Landman, J. W. (Hans) Niemantsverdriet, and Jannie C. Swarts, Inorg. Chem. 55 (2016) 1992
Abstract
Novel manganese(III)−ferrocenyl complexes of the type [Mn(FcCOCHCOR)3] have been synthesized, and the single-crystal structure of [Mn(FcCOCHCOCH3)3] was determined. X-ray photoelectron spectroscopy-determined binding energies of Fe(II) and Mn(III) in these complexes, and of Fe(II) in the free β-diketones, were found to relate linearly with the group electronegativities of each R group. This allows easy empirical prediction of Fe(II) and Mn(III) binding energies in these systems.
Involved partner
University of Bloemfontein
“Cu Model Catalyst Dynamics and CO Oxidation Kinetics Studied by Simultaneous in Situ UV–Vis and Mass Spectroscopy”
Yibin Bu, J. W. Hans Niemantsverdriet, and Hans O. A. Fredriksson, ACS Catal. 6 (2016) 2867
Abstract
The oxidation state of Cu nanoparticles during CO oxidation in CO + O2 gas mixtures was sensitively monitored via localized surface plasmon resonances. A microreactor, equipped with in situ UV–vis and mass spectrometry, was developed and used for the measurements. Cu nanoparticles of ∼30 nm average diameter were supported on optically transparent, planar quartz wafers. The aim of the study is 2-fold: (i) to demonstrate the performance and usefulness of the setup and (ii) to use the combined strength of model catalysts and in situ measurements to investigate the correlation between the catalyst oxidation state and its reactivity. Metallic Cu is significantly more active than both Cu(I) and Cu(II) oxides. The metallic Cu phase is only maintained under conditions where close to full oxygen conversion is achieved. This implies that kinetic measurements, aimed at determining the apparent activation energy for metallic Cu under realistic steady-state conditions, are difficult or impossible to perform.
“Methane, formaldehyde and methanol formation pathways from carbon monoxide and hydrogen on the (0 0 1) surface of the iron carbide χ-Fe5C2“
M. Olus Ozbek, J.W. (Hans) Niemantsverdriet, J. Catal. 325 (2015) 9-18
Abstract
Formation of CHx(O) monomers and C1 products (CH4, CH2O, and CH3OH) on C-terminated χ-Fe5C2(0 0 1) (Hägg carbide) surfaces of different carbon contents was investigated using periodic DFT simulations. Methane (CH4) as well as monomer (CHx) formation follows a Mars–van Krevelen-like cycle starting with the hydrogenation of surface carbidic carbon, which is regenerated by subsequent CO dissociation, while oxygen is removed as H2O. In cases where surface carbon is readily available, the apparent barrier for CH4 formation was found to be ∼95 kJ/mol. However, different rate-determining steps show that different propagation mechanisms may be possible for actual chain growth, depending on the carbon content of the surface. Hydrogen addition to CO forms formyl (HCO), which is a precursor for both H-assisted CO activation and oxygenate formation. Further hydrogenation of HCO yields adsorbed formaldehyde and methoxy, rather than hydroxymethyl (HCOH) that would give C–O bond splitting. Full hydrogenation to gas-phase methanol faces a high barrier, suggesting that CHxO species may be involved in higher oxygenate formation in a full Fischer–Tropsch mechanism or that the C–O bond does not break until the CHO fragment has been incorporated in a C2 species, a route for which precedents are available in the literature.
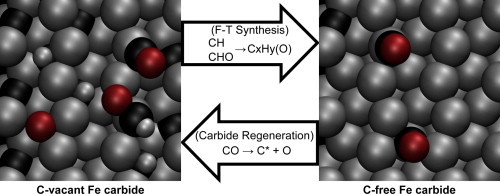
“Elementary reactions of CO and H2 on C-terminated χ-Fe5C2(0 0 1) surfaces”
M. Olus Ozbek, J.W. (Hans) Niemantsverdriet, J. Catal. 317 (2014) 158-166
Abstract
CO and H2 (co-)adsorption, direct and H-assisted CO activation, and surface carbon hydrogenation were investigated on C-terminated χ-Fe5C2(0 0 1) surfaces. Periodic DFT simulations at different surface carbon contents on the carbide surface showed that CO adsorbs preferably linearly on Fe top sites; CO and H2 adsorptions being stable. The perfect carbide surface favors carbidic carbon hydrogenation (i.e. CH formation), whereas carbon-free surface favors direct CO dissociation and restoration of the carbide structure. In partially carbon-vacant intermediate situations, both direct and H-assisted CO activations are energetically feasible, the latter being the preferred path. Considering CHx and CHxO species as initiators for different product types can explain the catalytic behavior and selectivity patterns of iron carbide catalysts. The catalytically active surfaces are concluded to be dynamic, where carbon atoms of the carbide surface participate in the surface reactions, and CO dissociation on vacant sites leads to restoration of the carbide structure.
Collaboration with our partners
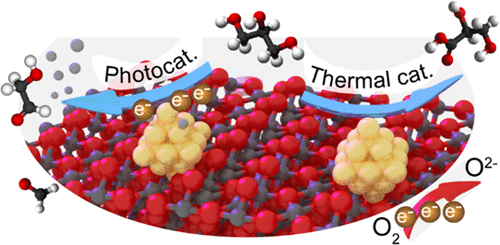
“Promotion Mechanisms of Au Supported on TiO2 in Thermal- and Photocatalytic Glycerol Conversion”
Y. Shen, A. Mamakhel, X. Liu, T. W. Hansen, T. Tabanelli, D. Bonincontro, B. B. Iversen, L. Prati, Y. (Yongwang) Li, J. W. (Hans) Niemantsverdriet, G. Hutchings, N. Dimitratos, A. Villa, R. Su, J. Phys. Chem. C 123 (2019) 19734-19741
Abstract
Catalytic glycerol conversion by means of either photon or thermal energy is of great importance and can be realized by metal supported on TiO2 systems. Although various procedures have been employed to synthesize efficient metal/TiO2 catalysts, the promotional mechanisms for both reactions are still unclear due to the lack of well-defined systems. Here, we have deposited gold nanoparticles on a series of highly crystalline anatase TiO2 substrates with different crystallite sizes (7, 12, 16, 28 nm) by both direct precipitation and sol-immobilization methods to examine the effect of metal deposition methods and TiO2 sizes on both photo- and thermal catalytic glycerol reforming. For photocatalytic H2 evolution from glycerol, optimum performance was observed for the Au supported on 12 nm TiO2 for both deposition methods. For thermal catalytic glycerol oxidation, all catalysts show a similar selectivity to glycerate (>70%) regardless of the TiO2 size and metal deposition method; however, the metal deposition method significantly influences the catalytic activity. In situ UV–vis spectrometry reveals that the optimized photocatalytic performance originates from enhanced charge transfer kinetics and a more negative Fermi level for proton reduction, whereas electrochemical analysis reveals that the promoted glycerol oxidation is caused by the enhanced oxygen reduction half-reaction.
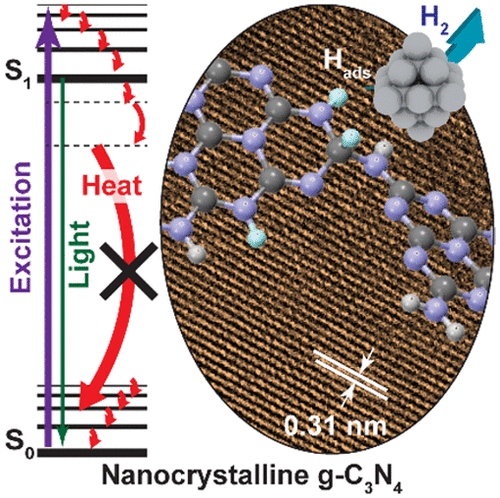
“Boosting Photocatalytic Hydrogen Production by Modulating Recombination Modes and Proton Adsorption Energy”
Y. Dai, Q. Bu, R. Sooriyagoda, P. Tavadze, O. Pavlic, T. Lim, Y. Shen, A. Mamakhel, X. Wang, Y. (Yongwang) Li, J.W. (Hans) Niemantsverdriet, B. B. Iversen, F. Besenbacher, T. Xie, J. P. Lewis, A. D. Bristow, N. Lock, R. Su, J. Phys. Chem. Lett. 10 (2019) 5381-5386
Abstract
PSolar-driven production of renewable energy (e.g., H2) has been investigated for decades. To date, the applications are limited by low efficiency due to rapid charge recombination (both radiative and nonradiative modes) and slow reaction rates. Tremendous efforts have been focused on reducing the radiative recombination and enhancing the interfacial charge transfer by engineering the geometric and electronic structure of the photocatalysts. However, fine-tuning of nonradiative recombination processes and optimization of target reaction paths still lack effective control. Here we show that minimizing the nonradiative relaxation and the adsorption energy of photogenerated surface-adsorbed hydrogen atoms are essential to achieve a longer lifetime of the charge carriers and a faster reaction rate, respectively. Such control results in a 16-fold enhancement in photocatalytic H2 evolution and a 15-fold increase in photocurrent of the crystalline g-C3N4 compared to that of the amorphous g-C3N4.
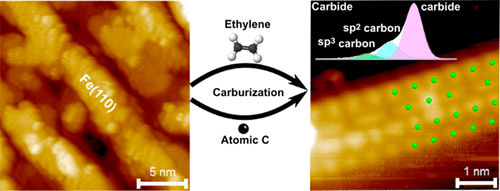
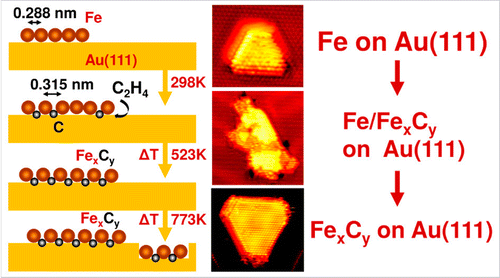
“Atomically Defined Iron Carbide Surface for Fischer-Tropsch Synthesis Catalysis: A molecular level picture and the effect of metal nanoparticles”
Y. Li, Z. Li, A. Ahsen, L. Lammich, G. J. A. Mannie, J. W. (Hans) Niemantsverdriet, J. V. Lauritsen, ACS Catal. 9 (2019) 1264-1273
Abstract
With the purpose of investigating the reactivity of Fe carbide as an active phase in Fischer–Tropsch catalysis, we studied the formation of a well-defined Fe carbide surface structure resulting from carbon exposure of an Fe film on Au(111). Using two different sources of carbon (C), namely atomic carbon and ethylene gas, we used synchrotron X-ray photoelectron spectroscopy (XPS) to show that a 6 ML Fe film readily converts into a well-defined and thermodynamically stable carbide phase. Scanning tunneling microscopy (STM) showed that the surface of the Fe carbide film is crystalline and is dominated by Fe(110)-like facets perturbed into a (2 × 2) periodic structure due to insertion of C in the interstitial sites. The reactivity of the carbide film toward CO, H2, and O2 was furthermore probed by XPS under vacuum conditions. While the pristine Fe carbide surface was unreactive toward hydrogen gas at 500 K, we interestingly found that CO dissociation from a preadsorbed monolayer of CO takes place already at low temperature. This observation points to an intrinsic activity of the Fe carbide phase where additional carbon originating from CO can be placed in the Fe carbide surface. The catalytic significance of the model catalyst surface presented here is that it can be seen as a stable Fe carbide phase with intrinsically vacant sites for additional C insertion at elevated pressure, and we propose that such additional C may act as active species in C–C coupling reactions during FTS. The studies pave the way for a better understanding of FTS processes on Fe-based catalysts on the basis of a well-defined model surface.
“In-situ probing photocatalytic C-C bond cleavage in ethylene glycol under ambient conditions and the effect of metal cocatalyst”
C. Li, X. Wang, A. Cheruvathur, Y. Shen, H. Xiang, Y. (Yongwang) Li, J.W. (Hans) Niemantsverdriet, R. Su, J. Catal. 365 (2018) 313-319
Abstract
Photocatalytic polyol conversion provides a green approach for the synthesis of value-added products. However, efficient and selective photocatalysts that can prevent unwanted full oxidation are still missing, mostly due to a lack of mechanistic understanding. Here we use ethylene glycol (EG) as model compound to study the reaction pathways in photocatalytic polyol dissociation under aerated conditions using in–situ vibrational spectroscopy coupled with mass spectrometry. On pristine TiO2, the presence of oxygen leads to the formation of formaldehyde via photocatalytic C_C bond cleavage, where the removal of photo-generated surface adsorbed proton (Hads+) in the form of water is the rate determining step (RDS). The photo-generated formaldehyde molecules subsequently convert into CO2 via complete oxidation by oxygen, or into paraformaldehyde by polymerization with water. A promotion effect is observed when noble metal (Au, Pt, Ag) nanoparticles (NPs) are used as cocatalysts. While Ag and Au selectively promote the formation of paraformaldehyde, the addition of Pt facilitates the complete oxidation of EG into CO2. By performing the reaction under a low oxygen partial pressure, we rationalize that Ag and Au NPs accelerate the polymerization of formaldehyde by providing water rapidly through direct oxidation of Hads oxidation, whereas Pt NPs supply water indirectly, in a pathway via H2 or formaldehyde oxidation.
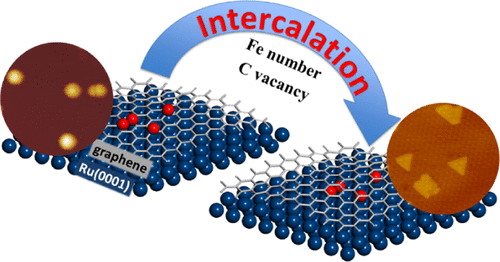
“Intercalation Mechanisms of Fe Atoms underneath A Graphene Monolayer on Ru(0001)”
Zhao, P. Ren, C. J. (Kees-Jan) Weststrate, Y. Xu, D. Cao, H. Xiang, J. Xu, Y. Yang, Y. (Yongwang) Li, J. W. (Hans) Niemantsverdriet, X. Wen, X. Yu, J. Phys. Chem. C 122 (2018) 22903-22910
Abstract
The intercalation process of iron atoms in the interface between graphene and Ru(0001) was systematically investigated both experimentally and computationally. Scanning tunneling microscopy and low-energy electron diffraction indicate that Fe intercalates at 700 K in the graphene/Ru(0001) system, where the graphene monolayer covers the whole substrate. An atomic-level understanding of the process is achieved using dispersion-corrected density functional theory (DFT) calculation. The results indicate that single-Fe atom intercalation causes only minor energy changes in the system. In contrast, the intercalation of a Fe dimer leads to a considerable drop in the total energy, more than twice the energy change in the case of the single-atom intercalation. In a sequential process, intercalation of the second Fe releases more energy, indicating that once the initial intercalation occurs, the subsequent process is thermodynamically more favored than the first. Combining the experimental observations with theoretical insights from the DFT calculations, we provide a clear picture of Fe intercalation into graphene/Ru(0001), which we believe is of interest to the field of interface and materials science and catalysis.
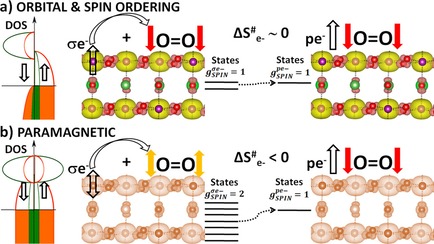
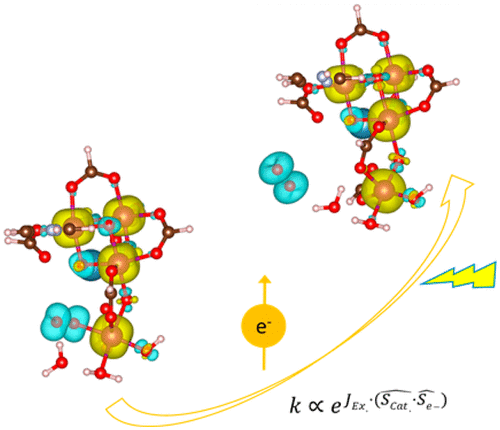
“Orbital Physics of Perovskites for the Oxygen Evolution Reaction”
R. Sharpe, J. Munarriz, T. Lim, Y. Jiao, J. W. (Hans) Niemantsverdriet, V. Polo, J. Gracia, Topics in Catalysis 61 (2018) 267–275
Abstract
The study of magnetic perovskite oxides has led to novel and very active compounds for O2 generation and other energy applications. Focusing on three different case studies, we summarise the bulk electronic and magnetic properties that initially serve to classify active perovskite catalysts for the oxygen evolution reaction (OER). Ab-initio calculations centred on the orbital physics of the electrons in the d-shell provide a unique insight into the complex interplay between spin dependent interactions versus selectivity and OER reactivity that occurs in these transition-metal oxides. We analyse how the spin, orbital and lattice degrees of freedom establish rational design principles for OER. We observe that itinerant magnetism serves as an indicator for highly active oxygen electro-catalysts. Optimum active sites individually have a net magnetic moment, giving rise to exchange interactions which are collectively ferromagnetic, indicative of spin dependent transport. In particular, optimum active sites for OER need to possess sufficient empty orthogonal orbitals, oriented towards the ligands, to preserve an incoming spin aligned electron flow. Calculations from first principles open up the possibility of anticipating materials with improved electro-catalytic properties, based on orbital engineering.
“Photocatalytic C-C bond cleavage in ethylene glycol on TiO2: A molecular level picture and the effect of metal nanoparticles”
Xianchi Jin, Chao Li, Chenbiao Xu, Dawei Guan, Ajin Cheruvathur, Yi Wang, Jian Xu, Dong Wei, Hongwei Xiang, J.W. (Hans) Niemantsverdriet, Yongwang Li, Qing Guo, Zhibo Ma, Ren Su, Xueming Yang, J. Catal. 354 (2017) 37–45
Abstract
Polyol conversion to value-added products is of great interest for the bio-diesel industry. Photocatalytic oxidation processes may offer a green approach for polyol conversion; however the lack of comprehensive mechanistic understanding from an interdisciplinary perspective limits or even misleads the design of highly selective and efficient photocatalysts for such process. Here we have studied the photocatalytic polyol conversion on pristine TiO2 and metal (Au, Pd, and Pt) nanoparticles (NPs) decorated TiO2 using ethylene glycol (EG) as the model compound. We have developed a mechanistic picture at molecular level by coupling in-situ surface science study on rutile (110) surface with in-situ vibrational-mass spectrometry study on TiO2 nanopowders. The C-C bond cleavage was found to be the only pathway in EG photo-conversion under deaerated conditions, leading to the formation of formaldehyde and hydrogen. We rationalized that the desorption of the surface adsorbed H (Hads) to be the rate determining step (RDS), making pristine TiO2 a poor photocatalyst that only catalyze the EG conversion at very low surface coverages. The addition of metal NPs on TiO2 surface promotes the desorption of Hads significantly, thus leading to an enhanced C-C bond cleavage performance at higher surface coverages that is more applicable.
“Analysis of the Magnetic Entropy in Oxygen Reduction Reactions Catalysed by Manganite Perovskites”
Jose Gracia, Julen Munarriz, Victor Polo, Ryan Sharpe, Yunzhe Jiao, Hans Niemantsverdriet, Tingbin Lim, ChemCatChem 9 (2017) 3358-3363
Abstract
Manganese oxides with a half‐metallic ground state are particularly active for oxygen reduction reactions (ORR). La0.67Sr0.33MnO3 (LSMO) perovskite is the archetypal example for compositions with a Curie temperature (TC) above room temperature and with a high intrinsic activity for the partial reduction of triplet‐state O2. The ferromagnetic (FM) character of the superexchange interactions in LSMO facilitates both charge and spin transport below 370 K. Other than the enhanced electronic conductivity, the reduced spin entropy seems to be relevant in oxygen catalysis because the magnetic ordering extends to the surface. The sign of the exchange interactions determines the adsorption of the triplet oxygen molecule with its spin antiparallel to the FM catalysts. Based on the transition‐state theory, we report that on LSMO, the hindrance resulting from the magnetic entropy for the initial reduction of O2 by two antiparallel electrons to diamagnetic intermediates (such H2O2) is minimum. On the other hand, the additional reduction of H2O2 to H2O, diamagnetic steps, prefers paramagnetic catalysts with higher magnetic entropy such as La0.4Sr0.6MnO3 to avoid spin accumulation.
“Photosystem II Acts as a Spin-Controlled Electron Gate during Oxygen Formation and Evolution”
Yunzhe Jiao, Ryan Sharpe, Tingbin Lim, Hans Niemantsverdriet, Jose Gracia, JACS 139 (2017) 16604–16608
Abstract
The oxygen evolution complex (OEC) of photosystem II (PSII) is intrinsically more active than any synthetic alternative for the oxygen evolution reaction (OER). A crucial question to solve for the progress of artificial photosynthesis is to understand the influential interactions during water oxidation in PSII. We study the principles of interatomic electron transfer steps in OER, with emphasis on exchange interactions, revealing the influence of delocalizing ferromagnetic spin potentials during the catalytic process. The OEC is found to be an exchange coupled mixed-valence electron-spin acceptor where its orbital physics determine the unique activity of PSII. The two unpaired electrons needed in the triplet O2 molecule interact with the high spin state of the catalyst via exchange interactions; the optimal ferromagnetic catalyst and the resulting radical intermediates are spin paired. As a result, the active center of the CaMn4O5 cofactor, stimulated by the driving potential provided by photons, works as a spin valve to accelerate the formation and release of O2 from diamagnetic H2O.
“Oxygen Evolution Reaction on Perovskite Electrocatalysts with Localized Spins and Orbital Rotation Symmetry”
Ryan Sharpe, Tingbin Lim, Yunzhe Jiao, J. W. (Hans) Niemantsverdriet, Jose Gracia, ChemCatChem 8 (2016) 37628-3768
Abstract
We have studied, by using ab initio calculations, the electronic properties of electro-catalysts for the oxygen evolution reaction (OER) with polarised density of states caused by localised spins in the d shell. Oxygen is a molecule in the triplet state (i.e. the outer electrons have parallel spins), which means that the spins localised in the p shell (↑O=O↑), the d shell and the conduction band electrons (t2gnegm) will couple through exchange interactions, which we think will provide favourable conditions for the OER. We compare the perovskites CaCu3Fe4O12 (CCF) and Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) with RuO2. CCF and BSCF both have fluctuating electronic structures accessible at room temperature that are linked to conducting spin-polarised density of states, equivalent to the paramagnetic state of covalent transition metal oxides with fine charge conductivity. CCF and BSCF both possess a considerable number of unpaired electrons localised in the inner d shell, high-spin configurations and competing inter-atomic exchange interactions. As a first approximation, the average fluctuation of the magnetisation in the metal atoms correlates linearly with the OER onset potential for the studied compositions. By linking the dynamics of the localised inner-electron spins to the conduction spins through exchange interactions, we can predict that other perovskites, such as Sr2Fe0.75Co0.25MoO6, will be OER active at room temperature, as they have similar electronic properties to CCF and BSCF.
“Mechanistic Insight into the Interaction Between a Titanium Dioxide Photocatalyst and Pd Co-catalyst for Improved Photocatalytic Performance”
Ren Su, Nikolaos Dimitratos, Jinjia Liu, Emma Carter, Sultan Althahban, Xueqin Wang, Yanbin Shen, Stefan Wendt, Xiaodong Wen, J. W. (Hans) Niemantsverdriet, Bo B. Iversen, Christopher J. Kiely, Graham J. Hutchings, and Flemming Besenbacher, ACS Catal. 6 (2016) 4239
Abstract
Understanding the co-catalyst/semiconductor interaction is of key importance for the design and synthesis of next generation photocatalytic materials for efficient hydrogen production and environmental clean-up applications. Here we investigate preformed Pd nanoparticles (NPs) supported on a series of anatase TiO2 having well-controlled but varying degrees of crystallinity and crystallite size, and explore their photocatalytic performance for H2 production and phenol decomposition. Whilst tuning the anatase crystallite size significantly influences the photocatalytic performance, varying the TiO2 crystallinity shows a negligible effect. Interestingly, the optimum quantum efficiency (~78%) for H2 evolution is achieved with anatase having medium crystallite size (~16 nm), whereas for phenol decomposition, a promotional effect is only observed for anatase with larger crystallite sizes (> 20 nm). Surface radical species and radical densities study reveal that the photogenerated charge carriers have been trapped at different sites depending on the crystallite size of anatase. Whilst the excited electrons are only trapped in bulk lattice sites in small anatase (<16 nm), larger anatase particles provide extra surface sites for charge trapping, which benefit charge storage and transportation to Pd surface sites, leading to a more efficient utilisation of charge carriers for photocatalysis. Additionally, Pd supported on medium-size anatase (~16 nm) hinders the formation of O2•- radicals on TiO2 surfaces, thus preventing unwanted re-oxidation of photogenerated H2.
“Monolayer Iron Carbide Films on Au(111) as a Fischer–Tropsch Model Catalyst”
Gilbère J.A. Mannie, Lutz Lammich, Yong-Wang Li, J.W. (Hans) Niemantsverdriet, Jeppe V. Lauritsen, ACS Catal. 4 (2014) 3255-3260
Editor’s choice = open access!
Abstract
Using scanning tunneling microscopy (STM), we characterize the atomic-scale details of ultrathin films of iron carbide (FexCy) on Au(111) synthesized as a potential model system for the active iron carbide phase in iron Fischer–Tropsch synthesis (FTS) catalysts. The experiments show that room-temperature exposure of Fe islands gas to C2H4 deposited on the clean Au(111) surface results in partly converted Fe/FexCy islands. Multistep flash-heating treatment of the partly converted Fe/FexCy islands at 523 and 773 K results in pure highly crystalline FexCy islands with in-plane nearest-neighbor distances of 0.315 ± 0.005 nm. On the basis of the atom-resolved STM data, we propose that C2H4 dissociates at Fe island edges, after which the carbon diffuses inward into the interstitial region between the Fe and the Au substrate to form an FexCy surface that may be a good starting point for the investigation of iron carbide surfaces present under FTS conditions.
Syngaschem staff has (co-)authored a number of other publications often based on previous activities:
Cornelis J.M. van der Ham, Furkan Işık, Tiny W.G.M. Verhoeven, J.W. (Hans) Niemantsverdriet, Dennis G.H. Hetterscheid, Catal. Today 290 (2017) 33–38
Paula Abril, M. Pilar del Rı́o, Cristina Tejel, Tiny W. G. M. Verhoeven, J. W. Hans Niemantsverdriet, Cornelis J. M. Van der Ham, Konstantin G. Kottrup, and Dennis G. H. Hetterscheid, ACS Catal. 6 (2016) 7872–7875
B. Caglar, M. Olus Ozbek, J. W. (Hans) Niemantsverdriet and C. J. (Kees-Jan) Weststrate, Phys. Chem. Chem. Phys. 18 (2016) 30117-30127
“Modeling the surface chemistry of biomass model compounds on oxygen-covered Rh(100)”
B. Caglar, J. W. (Hans) Niemantsverdriet, and C. J. (Kees-Jan) Weststrate, Phys. Chem. Chem. Phys. 18 (2016) 23888
Doreen Nabaho, J.W. (Hans) Niemantsverdriet, Michael Claeys, Eric van Steen, Catal. Today 275 (2016) 27
Doreen Nabaho, J.W. (Hans) Niemantsverdriet, Michael Claeys, Eric van Steen, Catal. Today 261 (2016) 17
“Hydrophilic Interaction Between Low-Coordinated Au and Water: H2O/Au(310) Studied with TPD and XPS”
Matthijs A. van Spronsen, Kees-Jan Weststrate, Angela den Dunnen, Maarten E. van Reijzen, Christine Hahn, and Ludo B. F. Juurlink, J. Phys. Chem. C 120 (2016) 8693
C.J. Weststrate, P. van Helden, J. van de Loosdrecht,J.W. Niemantsverdriet, Surf. Sci. 648 (2016) 60
“Oxygen Adsorption and Water Formation on Co(0001)”
A. C. (Ali Can) Kizilkaya, J. W. (Hans) Niemantsverdriet, and C. J. (Kees-Jan) Weststrate, J. Phys. Chem. C 120 (2016) 4833
“Ammonia Adsorption and Decomposition on Co(0001) in Relation to Fischer–Tropsch Synthesis”
A. C. (Ali Can) Kizilkaya, J. W. (Hans) Niemantsverdriet, and C. J. (Kees-Jan) Weststrate, J. Phys. Chem. C 120 (2016) 3834
J. Gracia, M. Escuin, R. Mallada, N. Navascues, J. Santamaria, Nano Energy 20 (2016) 20
“Modeling the Surface Chemistry of Sugars: Glycolaldehyde on Rhodium (100)”
Basar Caglar, M. Olus Ozbek, J. W. (Hans) Niemantsverdriet, and C. J. (Kees-Jan) Weststrate, J. Phys. Chem. C 119 (2015) 22915
Our Presentations
Oral
“Catalysis & Future Electricity Technology: What’s the Connection? ”
J.W. (Hans) Niemantsverdriet, Purdue University, West Lafayette, Indiana, USA, Jul 13, 2017
“Catalysis & Future Electricity Technology: What’s the Connection? ”
J.W. (Hans) Niemantsverdriet, Summer School Solids4Fun – TU Vienna, Waidhofen a/d Ybbs, Austria, Jul 6, 2017
“CO as an active spectator species in hydrocarbon conversions related to Fischer-Tropsch synthesis”
Kees-Jan (C.J.) Weststrate, Gilbère (G.J.A.) Mannie, and Hans (J.W.) Niemantsverdriet, 25th North American Catalysis Society Meeting, Denver, Colorado, USA, Jun 4-9, 2017
“Ultrahigh vacuum studies of the Fischer-Tropsch synthesis reaction”
C. J. Weststrate, D. Garcia Rodriguez, J.W. (Hans) Niemantsverdriet, NEVAC day 2017, Eindhoven, The Netherlands, May 12, 2017
“Syngas and Fischer-Tropsch Synthesis for Energy-Dense Liquid-Fuel Production in Energy Technology”
Hans Niemantsverdriet, Jose Gracia and Kees-Jan Weststrate , CCESC 2016 Conference, Madrid, Spain, Sep 7-9, 2016
“Model Systems for addressing Fundamental and Practical Questions in Heterogeneous Catalysis”
J.W. (Hans) Niemantsverdriet, CINF Summer School 2016 – Reactivity of nanoparticles for more efficient and sustainable energy conversion – IV, Gilleleje, Denmark, Aug 8-13, 2016
“Syngas as Essential Ingredient of Electricity Storage Technology: Electrolysis and Fischer-Tropsch Synthesis”
J.W. (Hans) Niemantsverdriet, Post-symposium on Catalysis for Syngas and Methanol Conversion of ICC-2016, Huairou/ Beijing, China, Jul 10-11, 2016
“Why Fischer-Tropsch synthesis needs carbon monoxide: the role of CO in the FTS chain growth mechanism”
C. J. Weststrate, J.W. (Hans) Niemantsverdriet, ICC-2016, Beijing, China, Jul 3-8, 2016
J.W. (Hans) Niemantsverdriet, TU Munich CRC Graduate Academy, Burghausen, Germany, Jun 5-8, 2016
“Hydrocarbon chemistry on cobalt: surface science investigations of the FT chain growth mechanism”
C.J. Weststrate, NGSC 11, Tromsø, Norway, Jun 5-9, 2016
“Surface science investigations of the Fischer-Tropsch reaction on cobalt”
C.J. Weststrate, J. W. (Hans) Niemantsverdriet, 251st ACS National Meeting, San Diego, California, USA, Mar 13-17, 2016
“Fundamentally understanding Fischer-Tropsch synthesis on cobalt: how experimental surface science can help”
C.J. Weststrate, J.W. (Hans) Niemantsverdriet, ECOSS-31, Barcelona, Spain, Aug 30 – Sep 4, 2015
“Fischer Tropsch Synthesis in CTL and GTL technology”
J.W. (Hans) Niemantsverdriet, Workshop, The Theory and Practice of Catalysis, TSRC, Telluride, Colorado, USA, Jul 6-10, 2015
“Reflections on Fischer Tropsch synthesis mechanisms and catalytic sites concepts”
J.W. (Hans) Niemantsverdriet, Syngas Convention 2 , Cape Town, South Africa, Mar 29 – Apr 1, 2015
“Heterogeneous Catalysis: Design and Characterization”
Autumn School on Fundamental Aspects of Catalysis, C*CHANGE, Cape Town, South Africa, Mar 26-28, 2015
“Fischer-Tropsch synthesis in coal-to-liquids: Old technology with a bright future?”
J.W. (Hans) Niemantsverdriet, 249th ACS National Meeting, Denver, Texas, USA, March 22-26, 2015
“Atomic scale iron carbide thin films on Au(111) – a model catalyst for Fischer-Tropsch catalysis research”
Gilbère J. A. Mannie, Lutz Lammich, Yong-Wang Li, J. W. (Hans) Niemantsverdriet, Jeppe V. Lauritsen, ECOSS-30, Antalya, Turkey, Aug 31 – Sep 5, 2014
“Surface science and computational modeling of iron and iron carbide Fischer-Tropsch catalysts”
J.W. Niemantsverdriet, 248th ACS National Meeting, San Francisco, California, USA, Aug 10-14, 2014
“Surface reactivity of iron and iron carbides in CO hydrogenation”
M.O. Ozbek, J.W. Niemantsverdriet, 248th ACS National Meeting, San Francisco, California, USA, Aug 10-14, 2014
“Atomic scale iron carbide thin films on Au(111) – a model catalyst for Fischer-Tropsch catalysis research”
Gilbère J. A. Mannie, Lutz Lammich, Yong-Wang Li, J. W. (Hans) Niemantsverdriet, Jeppe V. Lauritsen, ICSOS-11, Warwick, UK, Jul 21-25, 2014
“Iron carbide thin films synthesis studied by STM for fundamental iron Fischer-Tropsch catalysis research”
Gilbère J. A. Mannie, Alexander S. Walton, J. W. (Hans) Niemantsverdriet, Jeppe V. Lauritsen, NORDFORSK, Tromsø, Norway, Jun 2-3, 2014
“Nanoparticles as Model Systems for CO Hydrogenation Catalysts”
Hans Niemantsverdriet, Workshop on Nanoparticles in Reactive Environment, Marseille, France, Jan 27-29, 2014.
Posters
“Hydrogen production from short-chain alcohols using polymeric proton conductors”
F. Sapountzi, M. Tsampas, H.O.A. Fredriksson, J.M. Gracia, and J.W. Niemantsverdriet, ICE 2017, Copenhagen, Denmark, Jun 13-15, 2017
“Electricity to Fuels: Catalysis for Energy Storage”
C.J. Weststrate, F. Sapountzi, A.R. Vaccaro, J.W. Niemantsverdriet, Brussels Sustainable Development Summit 2015, Brussels, Belgium , Oct 19-20, 2015
“Thin FexCy films on Au(111) and Cu(111): exploring Fe Fischer-Tropsch model catalysts”
Gilbère J. A. Mannie, M. Rocío Bernal, Yong-Wang Li, J. W. (Hans) Niemantsverdriet, Jeppe V. Lauritsen, NORDFORSK, Lund, Sweden, Nov 24, 2014
“Phase transformation behavior of iron nanoparticles in Fischer-Tropsch synthesis”
Chenghua Zhang, M. (Emad) Dad, Kangkai Liu, Qiang Chang, J.W. (Hans) Niemantsverdriet, NCCC XV, Noordwijkerhout, The Netherlands, Mar 10-12, 2014