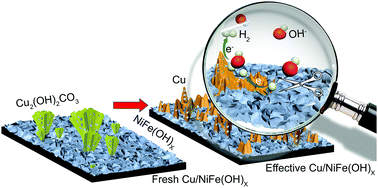
Spontaneous growth of copper dendrites stabilizes NiFe(OH)x electrocatalysts for hydrogen evolution for over 1000 hours
The team of Professor Ren Su and his students and coworkers at Soochow University and SynCat@Beijing has found an effective way to stabilize such materials, and they have demonstrated it by adding copper as a promoter to the hydroxide NiFe(OH)x. The copper initially forms malachite dendrites (Cu hydroxy carbonate, see the left part of the figure). During electrocatalysis, the mixed material restructures, such that copper metal dendrites form, which protect the active NiFe(OH)x phase from being hydrogenated to the metallic phase, which would cause loss of activity for hydrogen formation (Figure, right part).
Metallic Cu nanoflakes form spontaneously from the original malachite dendrites, and serve as antennae to collect extra hydrogen atoms from the neighboring NiFe(OH)x nanocrystals, thus avoiding their unwanted hydrogenation. The novel Cu/NiFe(OH)x cathode yields a durable high performance for water splitting over 1000 h when coupled with a standard NiFe-LDH anode for O2 production, and provide a promising system for commercial applications.
Syngaschem’s Foteini Sapountzi and Hans Niemantsverdriet were involved in the interpretation and discussion of these exciting results, and a joint publication was written for the journal Chemical Communications of the Royal Society of Chemistry (I.F.= 6.222) which appeared in April.
Reference
Guanming Shang, Yu Liu, Yajiao Li, Wei Qiao, Chao Wang, Yaru Li, Dongsheng Zhang, Foteini Sapountzi, Yong-Wang Li, Hans Niemantsverdriet, Mark H Rümmeli, and Ren Su; Copper dendrite stabilized NiFe(OH)x electrocatalyst for durable alkaline hydrogen evolution over 1000 h, Chemical Communications (April 2022)
DOI: https://doi.org/10.1039/D2CC01439D
Published on May 5, 2022
Figure: Durable Cu/NiFe(OH)x electrocatalyst for the hydrogen evolution reaction in alkaline media forms spontaneously under reaction conditions. The in situ generated Cu nanodendrites protect the NiFe(OH)x from being hydrogenated, giving it a more than 1000 h lifetime for high-performance water splitting when coupled with a NiFe-layered double hydroxide anode.