Hydrogen Adsorption on Cobalt Surfaces
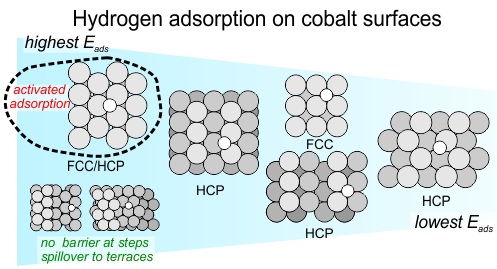
Fischer-Tropsch synthesis starts with adsorption of the reactants on the surface of the catalyst. Hydrogen atoms are required in many of the reaction steps that occur on the surface, but they arrive at the surface in the form of a hydrogen molecule. One of the important actions of the catalyst is to break the H-H bond to form adsorbed hydrogen atoms, the actual reactant needed.
In our recently published article in Journal of Catalysis, we describe the details of this process on single crystal cobalt model catalysts. Hydrogen adsorption and desorption turn out to be rather complex, and depends strongly on the structure of the cobalt surface. Step sites play an important role as they enhance H2 dissociation. Hydrogen atoms were found to bind most strongly on the most dense cobalt surface, contrary to the trend for atomic adsorbates which typically shows stronger adsorption on more open, and therefore more reactive, surfaces.
Published on January 12, 2022