Why is Syngaschem interested in Electrolysis?

Introduction
Syngas consists of carbon monoxide and hydrogen, CO and H2, and depending on the source, the H2/CO ratio may be too low for further conversion into methanol and synthetic fuels.
Applying the watergas shift reaction, CO + H2O = CO2 + H2, is the traditional way to produce more H2, but this goes at the cost of CO2. In situations where renewable electricity is available, e.g. from wind, solar, hydro or nuclear energy, electrolysis of water may become an attractive option.
Opportunities for Electrolysis
We see two major fields for future applications of electrolysis:
1) Coal-to-Liquids technology
If both green electricity and water are available, electrolysis of water generates H2 and O2. The former can be used to upgrade the syngas, while the latter can at least partly be used in the gasification of coal (oxygen generation by cryogenic air separation is expensive technology!)
The intermittency of wind and solar electricity can be a problem, because opportunities for storage are limited. Here electrolysis of water offers the option to generate H2 (and O2). If also a source of CO2 is available, syngas can be prepared, and conversion into liquids (methanol or hydrocarbons) offers a convenient storage medium of high energy density. It is even possible to co-electrolyse CO2 and H2O into CO+H2 and O2, creating the opportunity to produce syngas directly from buffer volumes of water and CO2. However, the latter process needs significant R&D before it is viable.
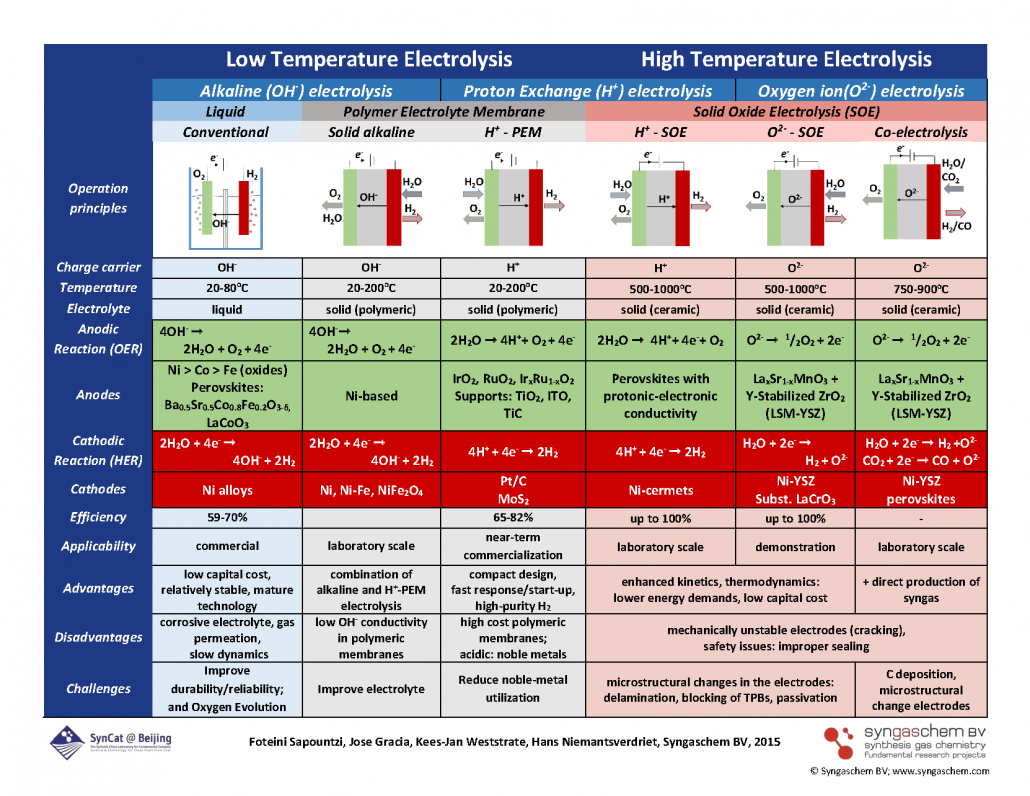
The scheme below summarizes the many opportunities and challenges in electrolysis. It may freely be downloaded and used, provided credit is given to the source: © Syngaschem BV; www.syngaschem.com