Iron Phosphide, a feasible replacement of Platinum in PEM electrolyzers
The main elements of this study are:
- A facile and novel synthesis route of high quality FeP nanoparticles is presented which enables preferential orientation of nanoparticles while it also omits the need of an intermediate iron reduction step.
- PEM water electrolysis experiments coupled to in-situ product analysis confirm that the observed currents indeed correspond to hydrogen production and exclude possible side reactions.
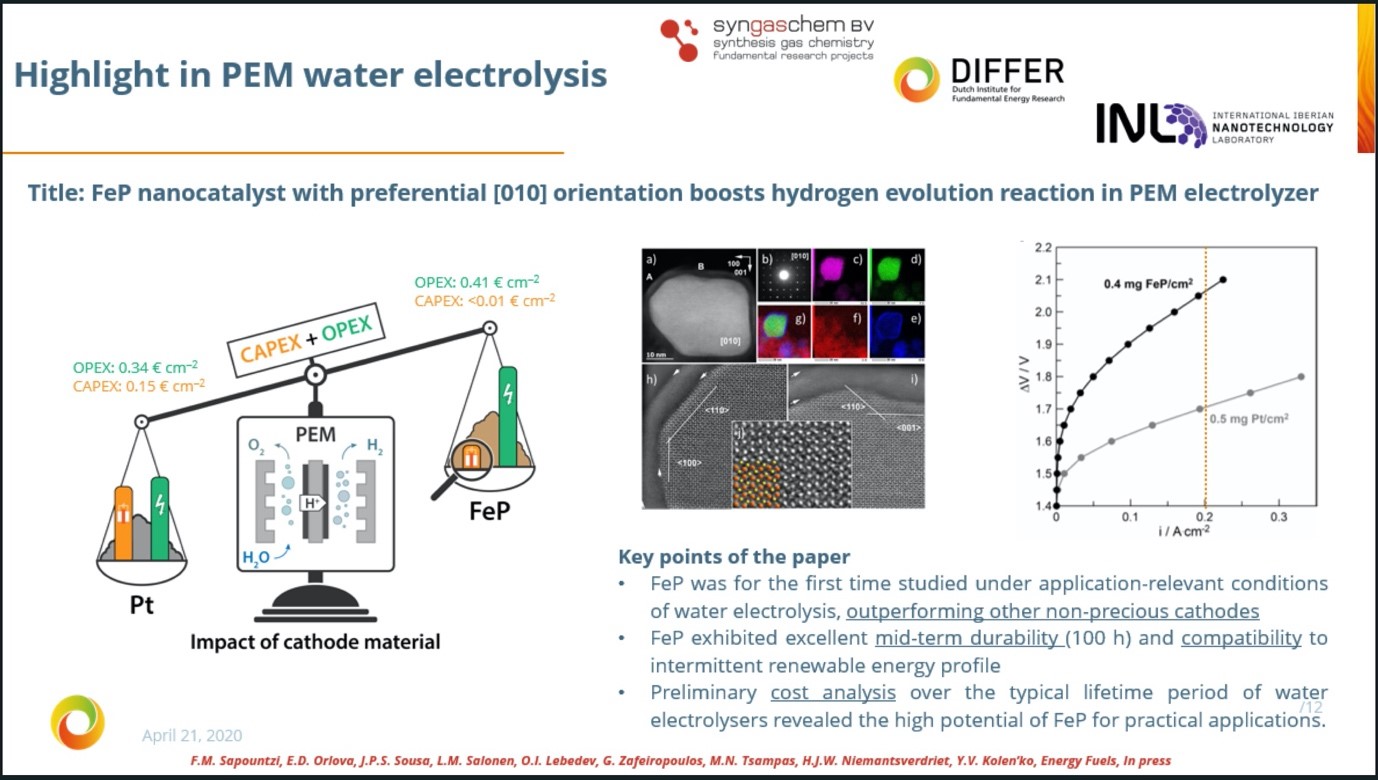
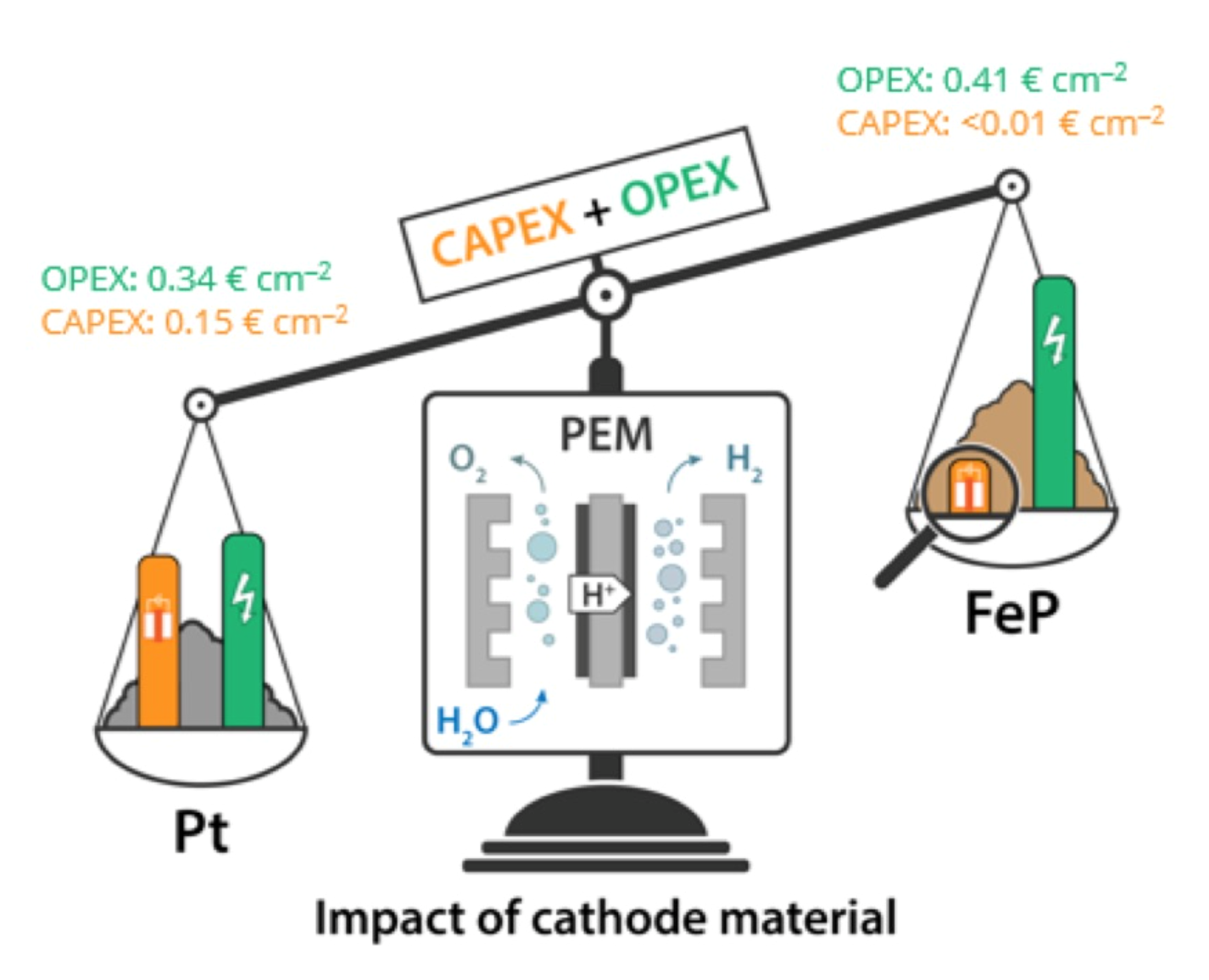
- Among the few existing studies where non-precious cathodes are employed in application-relevant full cells, our FeP cathode shows the best activity (among the ones operated at ambiente temperatures).
- The mid-term durability of our FeP cathode was demonstrated over operation at constant potential for 100 hours.
- Compatibility with intermittent renewables (e.g. solar and wind electricity) was also demonstrated via durability tests under variable power input.
- A preliminary cost analysis and comparison with state-of-the-art Pt cathodes shows that FeP holds great promise for practical applications.
This collaborative work of Syngaschem, DIFFER, International Iberian Nanotechnology Laboratory (INL, Braga, Portugal) and Laboratoire CRISMAT – CNRS (Caen, France), has been accepted for publication in the ACS journal Energy & Fuels. The article is now available online with DOI number: 10.1021/acs.energyfuels.0c00793 with a as title:
“FeP Nanocatalyst with Preferential [010] Orientation Boosts the Hydrogen Evolution Reaction in Polymer-Electrolyte Membrane Electrolyzer”
And authors:
Foteini M. Sapountzi, Elena D. Orlova, Juliana P.S. Sousa, Laura M. Salonen, Oleg I. Lebedev, Georgios Zafeiropoulos, Mihalis N. Tsampas, Hans Niemantsverdriet, Yury V. Kolen’ko
The slide below has been presented by Foteini Sapountzi in the on-line DIFFER meeting on April 21, 2020.
Published on April 22, 2020